Why is Kappa/lambda In-situ Hybridization Critically Acclaimed?

By admin
Detection Methods of Kappa/Lambda
The detection of Kappa/Lambda is commonly performed using four methods in the laboratory: in-situ hybridization, immunohistochemistry, flow cytometry, and PCR.
In-situ Hybridization
The Kappa/Lambda chain probe reagent is a specialized reagent used in the in-situ hybridization method. In order to find the κ:λ mRNA sequence more accurately and sensitively, it is intended to specifically target that gene. It is very helpful that this reagent can be used with a bigger range of materials. This makes it useful for many different tasks in molecular pathology and clinical diagnostics.
Flow Cytometry
Flow cytometry is a relatively straightforward process that involves the preparation and labeling of tissue samples before analysis using a machine. As part of this method, monoclonal fluorescent antibodies are used to label particular proteins on the cell surface and inside the cells. The target proteins can be found very specifically with this method. Also, individual biases are less likely to affect how the results are interpreted, which makes it more sensitive. However, it is important to note that fresh tissue samples that have not been fixed are necessary for flow cytometry analysis.
Immunohistochemistry
Immunohistochemistry (IHC) technology is a well-established technique with minimal equipment requirements. It is commonly used to label formalin-fixed paraffin-embedded (FFPE) samples. However, one limitation of IHC is the potential interference from mesenchyme immunoglobulins (Ig) due to the secretory nature of antibodies. This could change how specific the coloring data are. IHC can also make it hard to find immunoglobulins that are not present in large amounts. Even though these issues exist, IHC is still a useful method for studying and diagnosing because it lets us see where and how proteins are produced in tissue samples.
PCR
When employing the polymerase chain reaction (PCR) method, it is necessary to send the samples to a specialized molecular laboratory for testing. Nucleic acids are taken out of the samples as part of this process. It is important to keep in mind that PCR study does not tell you anything about tissues or cells. Its main goal is to find and increase specific DNA or RNA patterns. A lot of people in investigations and molecular biology use PCR to find and learn about genetic material.
It should be noted that accurate counting should be the primary factor for the detection of Kappa/Lambda,
Benefits of In-situ Hybridization of Kappa/Lambda
In molecular pathology, in-situ hybridization is a good way to get labeling data that is clear and accurate. When compared to immunohistochemistry, this method is better because it lets you see the parts of cells more clearly and exactly. The staining clarity achieved through in-situ hybridization allows for a more comprehensive analysis of the sample, leading to a more accurate diagnosis. This method is applicable to a wide range of sample types, making it a versatile tool in various diagnostic procedures.
Aside from that, in-situ hybridization is more sensitive and specific than other methods like flow cytometry and immunohistochemistry. This high sensitivity and specificity mean that in-situ hybridization can detect even minute changes in the cellular structure, making it a highly effective tool in diagnosing diseases at their earliest stages. Especially important for diagnosing diseases like plasma cell-related cancer and multiple myeloma, where finding them early can make a big difference in how well the patient does. In addition to its superior staining results and high sensitivity and specificity, in-situ hybridization also facilitates counting and determining whether light chain restricted proliferation has occurred. This trait is very important for correctly diagnosing a lot of different illnesses. We can get a better idea of how the disease is spreading with in-situ hybridization because it gives a clear and complete picture of the structure of the cells. This assists in making better treatment plans in the end. So, in-situ hybridization has benefits that go beyond its ability to diagnose. It also helps a lot with keeping diseases under control and treating them.
Kappa/Lambda Probe Kit (ISH) of Celnovte
The company Celnovte was established in 2010 and is a high-tech company that focuses on the study, creation, production, and distribution of precise tumor detection tools and materials.With a focus on early screening, accurate diagnosis, drug partner, and keeping track of tumors during treatment, the company wants to provide the best products and services. Celnovte has established a comprehensive industrial chain integrating R&D, production, sales, and service, aiming to become a leading provider of comprehensive solutions for tumor pathology diagnosis products both domestically and internationally. For instance, CNT360 Full-automatic IHC&ISH Stainer, CNT 620 Fully Automatic HE Drip-stainer and Coverslipper, and other high-quality instruments for diagnosis are very popular. The company has obtained ISO9001, ISO13485, and EU CE-ID certifications, demonstrating its commitment to quality and innovation.
Celnovte launches a Kappa/Lambda Probe Kit (ISH). When in-situ hybridization methodology is used, it can generate clear staining and superior performance compared to immunohistochemistry with the breakthrough of the MicroStackerTM microaggregate secondary antibody technology. It avoids background counting interference caused by non-specific staining of immunoglobulins in the immunohistochemical intercellular substance.
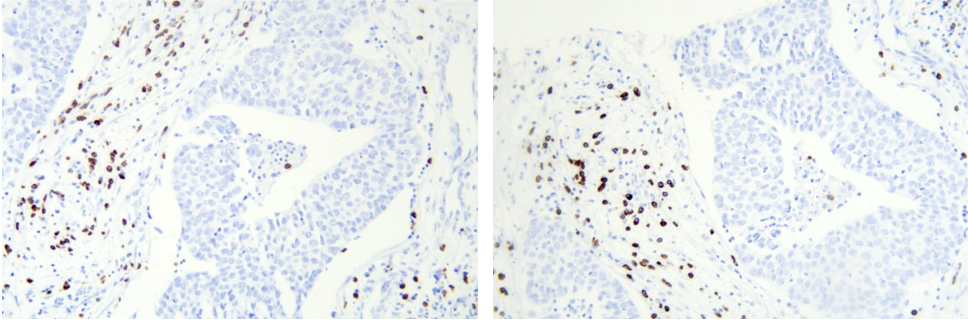
Lymphoma sample:
Kappa probe kit and Lambda probe kit
Application Scenario
κ and λ are two types of light chains on immunoglobulins. The ratio of plasma cells expressing different light chains κ:λ in normal lymph nodes is approximately 2:1. Restricted expression of kappa or lambda suggests monoclonal proliferation and tumor formation. The Kappa/Lambda in-situ hybridization detection project can detect the κ/λ ratio in plasma cells to confirm whether κ or λ clonal proliferation (i.e., restricted expression of that light chain) occurs in lymphoid tissue.
The Kappa/Lambda in-situ hybridization detection project is widely used in the diagnosis of multiple myeloma. It is also used in the diagnosis of plasma cell-related diseases such as extranodal lymphoma with plasma cell differentiation, monoclonal gammopathy of undetermined significance (MGUS), AL amyloidosis, Waldenstrom macroglobulinemia, diffuse large B-cell lymphoma (DLBCL), mantle cell lymphoma (MCL), chronic lymphocytic leukemia (CLL)/small lymphocytic lymphoma (SLL), marginal zone lymphoma (MZL), Burkitt lymphoma (BL), and other common subtypes of non-Hodgkin lymphoma, Hodgkin lymphoma, follicular center lymphoma, precursor B-cell leukemia, or acute lymphoblastic leukemia.
Principle of the Kappa/Lambda Probe Kit (ISH)
The probing process in the Kappa/Lambda in-situ hybridization detection project is based on the specific hybridization recognition of the probe labeled with digoxigenin (DIG) derived from plants. Through the use of a signal cascade including secondary antibodies, this innovative approach enables the sensitive and precise detection of kappa/lambda cells. This method may reliably and accurately detect multiple myeloma and other plasma cell-related diseases by focusing on the kappa/lambda mRNA sequences. An accessible and practical molecular pathology tool, the HRP-DAB chromogenic system enables observation of staining data under a light microscope. With its practical clinical and pathological value, this technology, including the widely adopted EBER in-situ hybridization, has become the gold standard for diagnosing tissue pathology EBV infection. All things considered, the Kappa/Lambda in-situ hybridization detection project provides very specific and sensitive results, which aid in the identification and study of lymphomas and associated diseases.
Features of the Kappa/Lambda Probe Kit (ISH)
High sensitivity: Targets kappa/lambda mRNA sequences and utilizes an innovative polymer secondary antibody chromogenic system.
High specificity: Utilizes DIG labeling derived from plants and optimized probe sequences.
Good stability: Follows SOP production processes and exhibits good thermal stability.
Easy to use: Provides stable validation protocols, with only two additional steps compared to immunohistochemistry, making it convenient to use.
Easy to interpret: Provides positive control images for accurate staining localization.
More flexibility: Available as both manual and automated reagent kits, compatible with dual staining.
Experimental procedure
Enzyme digestion – Probe hybridization – Primary antibody incubation – Secondary antibody incubation – DAB chromogenic staining
In this procedure, only enzyme digestion and probe hybridization steps is added compared to immunohistochemistry, which is more convenient to carry out!