SPOTLIGHT ON American Association for Cancer Research (AACR) Annual Meeting 2025
2025-04-25
By admin
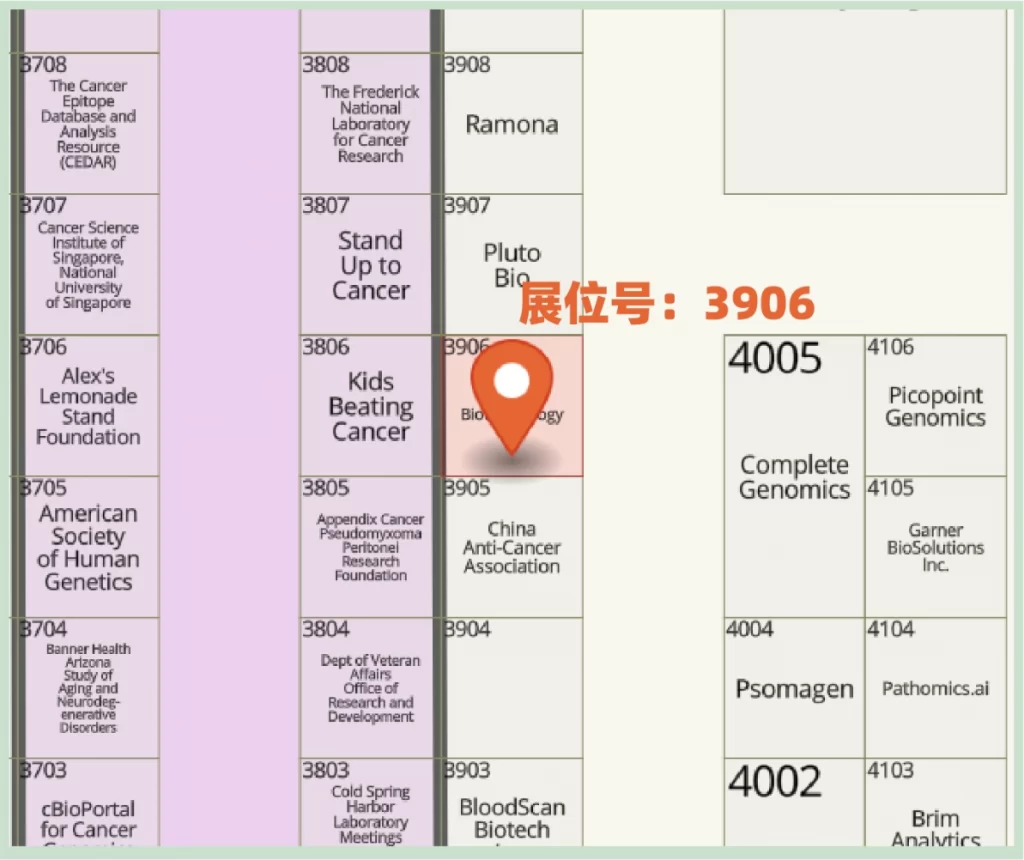
Celnovte Biotech Booth Number: 3906
About the AACR Annual Meeting
The 2025 annual meeting of the American Association for Cancer Research (AACR), 116th in its annual series, will be hosted from April 25 to 30 in McCormick Place Convention Center in Chicago, USA. The world-class conference is a leading conference on cancer research and brings together leading professionals, scholars, and the world’s leading oncology experts to exchange the newest advances in basic science, clinical practice, translational research, and new technologies
The AACR Annual Meeting is the oldest and most extensive scientific conference focused on cancer research. It covers every aspect of oncology. It encourages knowledge sharing among worldwide cancer researchers. It also sparks creative discoveries and offers valuable learning opportunities for young scientists. Moreover, AACR raises public understanding of cancer. It supports the global effort against the illness. As the leading source on cancer research trends, the 2025 AACR Annual Meeting will include milestone discoveries, novel therapies, and precision medicine breakthroughs. These will provide vital insights for global cancer research and care.
Exhibition Highlights
CNT320RX Fully Automated Immunohistochemistry (IHC) Staining System
- Provides excellent staining accuracy and flexible detection techniques to aid advanced cancer studies.
- Helps scientists explore new research areas with consistent and dependable results.
Class III Medical Device Registered Reagents
- Complete solutions for breast cancer diagnostics.
|
Product Name |
Registration Number |
Intended Use |
|
HER2 Antibody Reagent (Immunohistochemistry) |
NMPA 20243402106 |
Companion diagnostic for trastuzumab (Herceptin). |
|
Hormone Receptor Antibody Reagent (Immunohistochemistry) |
NMPA 20213400742 |
Semi-quantitative detection of estrogen receptors in 10% neutral buffered formalin-fixed, paraffin-embedded human breast cancer tissue sections. |
|
Progesterone Receptor Antibody Reagent (Immunohistochemistry) |
NMPA 20233400487 |
Semi-quantitative detection of progesterone receptors in 10% neutral buffered formalin-fixed, paraffin-embedded tissue sections. |
|
HER2 Gene Amplification Detection Kit (Fluorescence In Situ Hybridization) |
NMPA 20243400374 |
Companion diagnostic for trastuzumab (Herceptin). |
About Celnovte Biotech
Celnovte Biotechnology Co., Ltd. based in Zhengzhou, China, focuses on creating and manufacturing diagnostic reagents and tools. The company runs a branch for instrument development in Shenzhen. It also has a research center for scientific products in Suzhou and a reagent facility in Maryland, USA. Its Chinese sites meet NMPA and GMP requirements. They hold ISO 13485, ISO 9001, and CE certifications. Now, Celnovte maintains a broad global pathology network. It serves leading hospitals in China and more than 10 countries worldwide.
Celnovte concentrates on developing, producing, and selling pathology diagnostic reagents and equipment. It emphasizes advanced immunohistochemistry (IHC) and in situ hybridization (ISH) products. Key advancements include:
- Microstacker™ Secondary Antibody Detection System: Delivers outstanding precision and clarity for accurate diagnostics.
- Polystacker™ Technology: Shortens intraoperative frozen IHC testing to only 10 minutes.
- super-ISH™ mRNA In Situ Hybridization Technology: Allows exact detection of target mRNA at single-molecule and single-cell levels.
Celnovte’s diverse range of pathology diagnostic tools includes:
- Fully automated HE staining and coverslipping systems
- Fully automated special staining and coverslipping systems
- Fully automated liquid-based cytology IHC staining systems
- High-throughput IHC staining systems
- Digital pathology scanners
Since its debut in 2018, Celnovte has installed over 600 fully automated IHC staining systems globally. As a pioneer in innovation, Celnovte is committed to advancing precision medicine with state-of-the-art pathology diagnostics.