Recommendation of antibodies: TROP2-immunohistochemistry

By admin
 What is TROP2?
What is TROP2?
Definition and Structure of TROP2
TROP2 (immunohistochemistry) is a transmembrane protein that exists on the surface of human trophoblast cells and tumor cells.
TROP2 is a popular molecular target for cancer treatment, and antibody-drug conjugates (ADCs) developed against TROP2 have been approved and marketed. For example, trastuzumab deruxtecan is an ADC used for the treatment of patients with triple-negative breast cancer (TNBC).
At the recently concluded 2023 ASCO Annual Meeting, TROP2 ADC drugs, Datopotamab deruxtecan and SKB264, demonstrated high therapeutic potential in first-line and later-line treatments for non-small cell lung cancer (NSCLC) (ASCO ADC Session | TROP2 ADC Shines).
According to the management regulations of the National Health Commission, targeted anti-tumor drugs with specific targets must undergo corresponding target testing before use. For drugs targeting the TROP2 target, immunohistochemistry testing in the pathology department can provide precise medication guidance.
Role of TROP2 in Cellular Processes
TROP2 is involved in diverse cellular processes such as cell proliferation, migration, and adhesion. It functions by interacting with other cell surface molecules, influencing pathways that regulate the cell cycle and apoptosis. TROP2 has been shown to facilitate the communication between cells and their surrounding environment, making it a crucial player in maintaining cellular integrity and function.
Importance of TROP2 in Cancer
Overexpression of TROP2 in Tumors
Research has revealed that TROP2 is frequently overexpressed in various forms of cancer, including breast, lung, and colorectal carcinomas. This overexpression is closely associated with aggressive tumor behavior and poor prognosis. Elevated levels of TROP2 contribute to increased cellular proliferation and metastasis, which highlight its significance in oncogenesis.
Mechanisms of TROP2 in Cancer Progression
TROP2 facilitates cancer progression through several mechanisms. It promotes epithelial-mesenchymal transition (EMT), enhancing the invasive capabilities of cancer cells. Moreover, TROP2 activates the Akt and ERK signaling pathways, which are crucial for cell survival and proliferation. These mechanisms collectively enable tumors to grow and spread more efficiently, underscoring the potential of TROP2 as a therapeutic target.
Diagnostic and Prognostic Value of TROP2
TROP2 protein and pathological testing
Studies have shown that TROP2 is overexpressed in various malignant tumors, such as cervical cancer (89%), urothelial carcinoma (83%), papillary thyroid carcinoma (83%), breast cancer (80%), squamous cell lung carcinoma (75%), endometrial cancer (72%), prostate cancer (71%), colon cancer (68%), adenocarcinoma of the lung (64%), epithelial ovarian cancer (59%), gastric cancer (56%), and pancreatic cancer (55%), and is associated with poor prognosis and increased risk of metastasis.
It is worth mentioning that several clinical studies related to drugs targeting TROP2 are currently underway. As of June 2023, there are 33 global drug developments targeting TROP2, 46 Chinese drug evaluations, 96 global clinical trials, and 27 Chinese clinical trials targeting TROP2 (data source: PharmCloud).
Biomarker Potential in Oncology
Early Detection and Diagnosis
TROP2 serves as a valuable biomarker for the early detection and diagnosis of cancer. Its high expression levels in malignant tissues compared to normal tissues make it an excellent candidate for diagnostic assays. Detecting TROP2 can aid in identifying cancers at an earlier stage, potentially improving patient outcomes through timely intervention.
Predictive Value for Treatment Outcomes
The expression level of TROP2 can also predict treatment responses in cancer patients. Studies have shown that tumors with high levels of TROP2 may respond differently to certain chemotherapies and targeted therapies. Thus, measuring TROP2 expression can help oncologists tailor treatment plans to achieve better efficacy and minimize adverse effects.
Therapeutic Targeting of TROP2
Development of TROP2-Targeted Therapies
Monoclonal Antibodies Against TROP2
Monoclonal antibodies that specifically target TROP2 have been developed to combat cancers overexpressing this protein. These antibodies bind to the extracellular domain of TROP2, inhibiting its function and triggering immune-mediated destruction of cancer cells. Clinical trials are underway to evaluate their safety and efficacy in various cancer types.
Antibody-Drug Conjugates (ADCs) Targeting TROP2
Antibody-drug conjugates (ADCs) targeting TROP2 are a promising therapeutic approach. ADCs consist of a monoclonal antibody linked to a cytotoxic drug, allowing for targeted delivery of the drug to cancer cells expressing TROP2. This method minimizes damage to normal tissues while effectively eliminating tumor cells.
Clinical Trials and Emerging Treatments
Several clinical trials are investigating new treatments targeting TROP2. Early results indicate that these therapies can reduce tumor size and prolong survival in patients with advanced cancers. Emerging treatments aim to enhance the specificity and efficacy of TROP2-targeted therapies, offering hope for improved cancer management in the future.
Future Directions for TROP2 Research
Potential Challenges and Limitations
Drug Resistance Issues
One potential challenge in targeting TROP2 is the development of drug resistance. Tumors may evolve mechanisms to evade the therapeutic effects of TROP2-targeted treatments. The main goal of research is to figure out these resistance routes and come up with combination treatments that can beat them.
Specificity and Safety Concerns
Ensuring the specificity and safety of TROP2-targeted therapies is critical. Off-target effects on healthy tissues can lead to adverse events. More study is being done to improve these treatments so that they are more accurate at killing cancer cells while sparing healthy cells. This will make them safer.
Promising Avenues for Future Studies
In the future, researchers may look into new ways to make TROP2-targeted medicines work better. These treatments might work better when used with other types of treatment, like immunotherapy or radiation. Finding signs that can predict how well TROP2-targeted drugs will work will also help tailor treatment to each patient.
The Impact of Understanding TROP2 on Cancer Treatment Strategies
Targeted therapies have changed a lot since scientists figured out what part TROP2 plays in cancer. Researchers have come up with new ways to treat cancer that are more exact and effective by taking advantage of its increase in tumors. More study into TROP2 is likely to lead to even more advanced treatment plans, which will have a big effect on the future of cancer.
On June 27, 2022, ASCO published an updated guideline on the biological markers for systemic treatment of metastatic breast cancer in the Journal of Clinical Oncology. Based on current practice, the recommendation changed how to find molecular markers for metastatic breast cancer. One of these markers is the expression of TROP2.
Currently, the expression of TROP2 protein is mainly detected using immunohistochemistry.
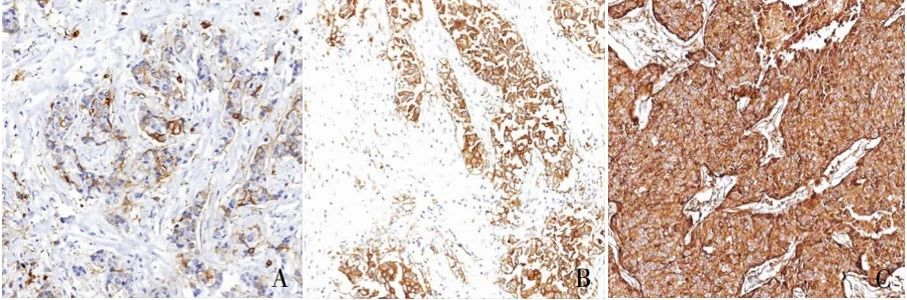
Example of TROP2 immunohistochemical staining scoring using the SP method: A. 1+, weak staining in the cell membrane or cytoplasm of >10% tumor cells; B. 2+, moderate staining in the cell membrane or cytoplasm of >10% tumor cells; C. 3+, strong staining in the cell membrane or cytoplasm of >10% tumor cells.
Celnovte Biotech, established in 2010, is a high-tech enterprise specializing in the research, development, production, and sales of precision diagnostic instruments and reagents for tumors. With a strong focus on tumor pathology, Celnovte has established a comprehensive system that adheres to GMP requirements, including production, quality control, and research and development.
Celnovte offers a comprehensive range of immunohistochemistry chemical reagents, including those that can be used to detect TOP 2 protein. With expertise in immunohistochemistry research and development, the company provides over 460 primary antibodies, secondary antibody detection systems, and more than 200 immunohistochemistry quality control products. Their products like Immune Chromogenic Reagent and MicroStacker™ Plus HRP-Polymer Detection Kit can all provide efficient solutions for immunohistochemistry. Celnovte also offers fully automated staining platforms, ensuring high-quality staining results for customers. These immunohistochemistry chemical reagents are designed to support precise diagnostic applications in the field of tumor pathology. With a commitment to quality, innovation, and service, Celnovte aims to be a leading provider of immunohistochemistry solutions for tumor pathology diagnosis products.
RELATED PRODUCTS







 What is TROP2?
What is TROP2?