Recommendation of antibodies: 2023 CSCO guidelines add detection target Claudin18.2.
 2024-06-18
2024-06-18
By admin
Claudin18.2 protein, with its high expression rate in gastric and pancreatic cancer tumors, holds great promise as an ideal target. It is considered to be a potential second important target in the field of gastric cancer, following HER2. The high expression of Claudin18.2 in these tumors makes it an attractive candidate for targeted therapies. Researchers and clinicians are eagerly anticipating the development of Claudin18.2-targeted treatments, as they have the potential to significantly improve outcomes for patients with gastric and pancreatic cancer. The identification of Claudin18.2 as a potential target opens up new avenues for precision medicine and personalized treatment approaches in these malignancies.
Introduction of Claudin18.2
Expression of Claudin18.2
Claudin18.2 protein is a highly selective marker protein that is highly restricted in its expression in normal tissues, with almost no expression. It is not expressed in the gastric stem cell zone capable of differentiating into gastrointestinal epithelial cells, but only expressed in differentiated gastric mucosal epithelial cells, and its expression level is very limited.
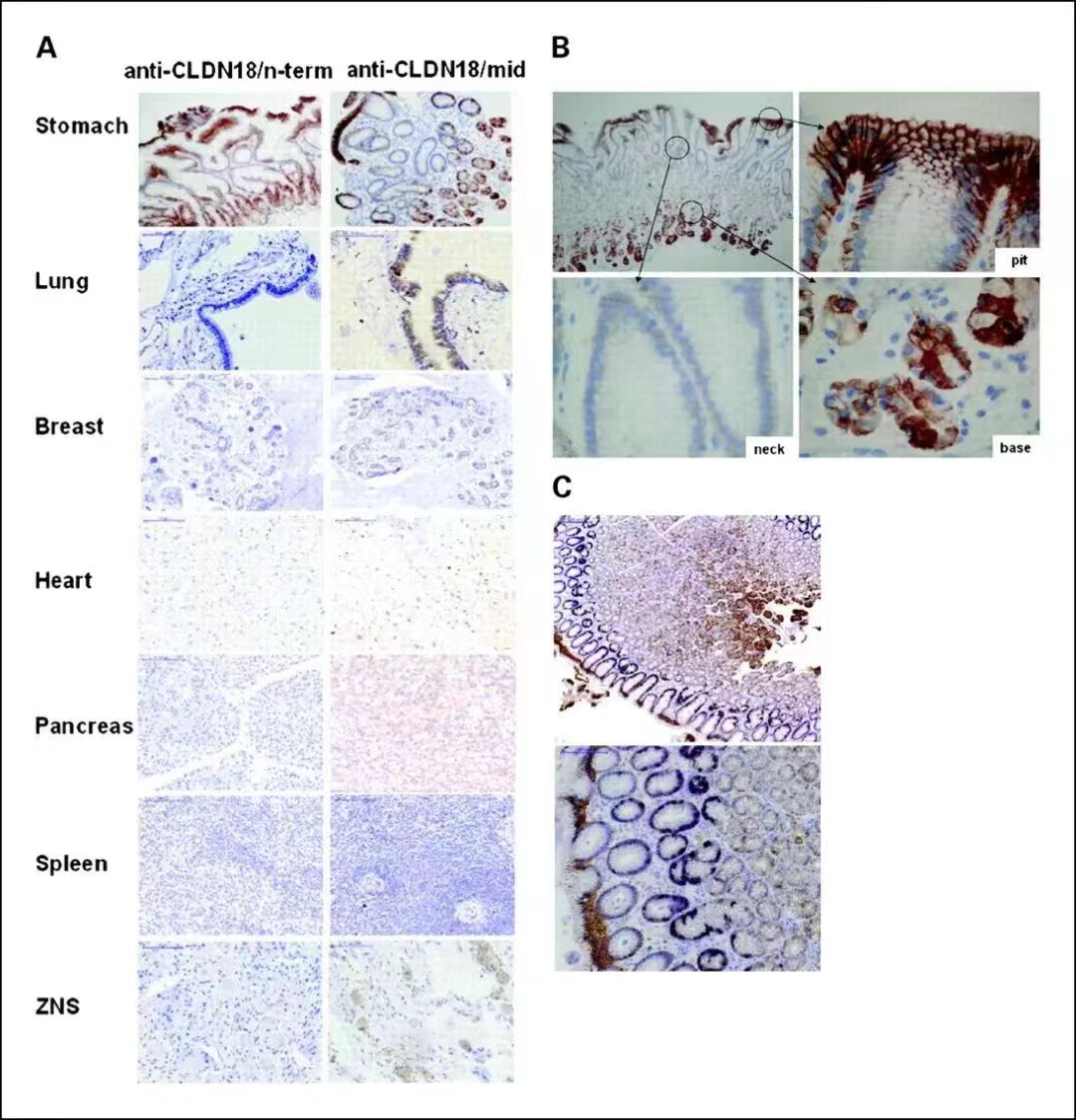
Expression of Claudin18.2 in normal tissues
The relationship between Claudin18.2 and tumors:
Abnormal expression of Claudin18.2 can lead to structural damage and functional impairment of epithelial and endothelial cells, playing an important pathogenic role in the invasion and metastasis of various epithelial-derived tumors. It frequently undergoes abnormal changes during the occurrence and development of various malignant tumors.
The Claudin18.2 gene can also be abnormally activated and selectively and stably expressed in specific tumor tissues, participating in the proliferation, differentiation, and migration of tumor cells. This makes it a potential effective molecular target for anti-tumor drugs.
Data analysis has revealed that Claudin18.2 can be expressed in various types of cancers under pathological conditions. The expression rate of Claudin18.2 in gastric cancer is approximately 70%, making it a prominent target in this disease. In pancreatic tumors, Claudin18.2 expression is observed in around 50% of cases, highlighting its relevance in this challenging cancer type. Additionally, Claudin18.2 expression has been detected in approximately 30% of esophageal cancer cases. Furthermore, Claudin18.2 expression has been reported in breast cancer, colon cancer, liver cancer, ovarian cancer, and lung adenocarcinoma. These findings suggest that Claudin18.2 may have a broader role as a potential therapeutic target across multiple cancer types, providing opportunities for the development of targeted therapies in these malignancies.

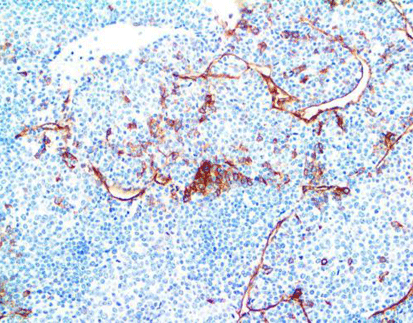
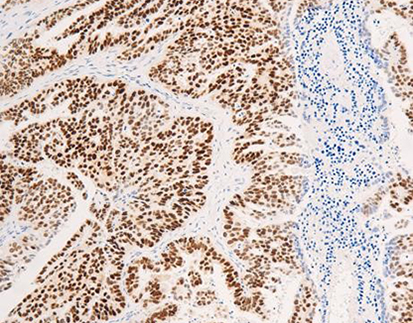
Claudin18.2 expression in tumor tissues: A. Gastric cancer tissues (Ⅰ, Ⅱ, Ⅲ, Ⅳ), gastric cancer metastasis to the ovaries (Ⅴ, Ⅵ); B. Esophageal adenocarcinoma (Ⅰ, Ⅱ) and mucinous ovarian cancer (Ⅲ, Ⅳ).
2023 CSCO gastric cancer diagnosis and treatment guidelines add Claudin18.2 testing recommendation
Molecular testing section:
Immunohistochemical detection of Claudin18.2 is classified as a level III recommendation for molecular testing in patients with metastatic gastric cancer, with an evidence level of 2B. An additional annotation is added: For advanced or recurrent gastric cancer patients who have failed standard treatment, Claudin18.2, FGFR2, C-MET, NTRK gene, and other biomarker testing can be performed to identify potential therapeutic targets.
First-line targeted therapy section:
The phase III SPOTLIGHT clinical trial has provided valuable data on the use of Zolbetuximab, a monoclonal antibody targeting Claudin18.2, in combination with chemotherapy for the treatment of advanced gastric cancer patients who are positive for Claudin18.2 and negative for HER2. This trial aimed to evaluate the efficacy and safety of Zolbetuximab in improving outcomes for these patients. The results of the trial will contribute to our understanding of the potential benefits of targeting Claudin18.2 in the treatment of advanced gastric cancer and may pave the way for the development of more effective therapeutic strategies in the future.
Specific content of the 2023 CSCO guidelines:
Claudin18.2 is moderately to highly expressed in approximately 40% of gastric cancer patients. In the phase III randomized controlled SPOTLIGHT study [s4], first-line treatment with Claudin18.2 monoclonal antibody Zolbetuximab in combination with mFOLFOX6 chemotherapy was compared to mFOLFOX6 chemotherapy alone in Claudin18.2-positive and HER2-negative advanced gastric/esophagogastric junction cancer patients. The study showed improved median progression-free survival (10.61 months vs 8.67 months, HR=0.751, P=0.0066) and overall survival (18.23 months vs 15.54 months, HR=0.750, P=0.0053) in the combination therapy group.
In a study initiated by researchers [5], 28 patients with standard treatment-refractory Claudin18.2-positive gastric cancer received CAR-T cell therapy, achieving an overall response rate (ORR) of 57.1%. Among 18 patients who had previously failed second-line treatment, the ORR was as high as 61.1%, with a median progression-free survival of 5.4 months and overall survival of 95 months. In another phase Ib study [s6] involving 14 patients with Claudin18.2-positive gastric cancer who had previously failed second-line treatment, the ORR reached 57.1%, with a median progression-free survival of 5.6 months and overall survival of 10.8 months. These results show significant improvement compared to existing third-line treatments, and confirmatory studies are currently underway for validation.
Research progress in Claudin18.2 targeted therapy:
Currently, multiple immunotherapy strategies targeting Claudin18.2 have been developed, including monoclonal antibodies (mAbs), bispecific antibodies (BsAbs), chimeric antigen receptor T-cell (CAR-T) therapy, and antibody-drug conjugates (ADCs). According to statistics, there are nearly 56 ongoing global projects in this field, covering indications such as solid tumors, pancreatic cancer, gastric cancer, esophageal cancer, esophageal adenocarcinoma, biliary tract cancer, gallbladder cancer, lung adenocarcinoma, and colon cancer.
Celnovte self-developed Claudin18.2 rabbit monoclonal antibody
Celnovte Biotech, established in 2010, is a high-tech enterprise specializing in the research, development, production, and sales of precision diagnostic instruments and reagents for tumors. With a strong focus on quality, innovation, and service, Celnovte has built a comprehensive system that complies with GMP requirements, including clean production workshops and advanced research and production equipment. The company holds ISO9001, ISO13485, and EU CE-ID certifications, positioning it as a leader in the field of tumor pathology reagents and instruments. Celnovte aims to become a benchmark technology innovation enterprise in the industry, contributing to scientific and technological advancements in life sciences and medicine. With a complete industrial chain and a wide range of products, including immunohistochemistry reagents, Celnovte strives to provide comprehensive solutions for tumor pathology diagnosis, making it a renowned provider both domestically and internationally. The selection of Claudin18.2 antibodies is crucial for diagnostic results as current target screening methods used in existing therapies are based on immunohistochemistry. They also produce high-quality products like H&E Stains and primary antibody facilitating diagnosis and treatment processes.
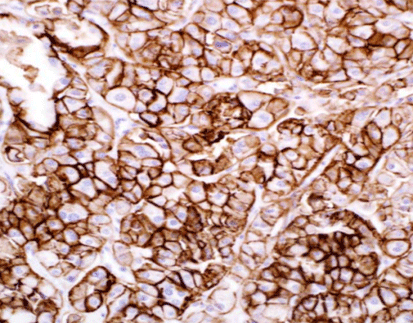
Celnovte’s Claudin18.2 (C7X3) rabbit monoclonal antibody exhibits strong specificity, high affinity, and high detection sensitivity. By utilizing rabbit monoclonal antibody development technology and engineered antibody expression technology, the antibody possesses enhanced stability.
Claudin18.2 (C7X3) rabbit monoclonal antibody demonstrates excellent staining results in various cancer tissues such as gastric cancer, breast cancer, esophageal cancer, and colon cancer. It accurately localizes positive signals with a clean background and can accurately identify tissues with low expression.

The Claudin 18.2 protein is encoded by the CLDN18 gene, located on chromosome 3q22.3. It belongs to the Claudin protein family and is often associated with occludin, playing a crucial role as a component of tight junctions. Normally, Claudin 18.2 protein is not expressed in normal tissues.
Clinical applications:
- It is primarily used for the diagnosis of digestive tract tumors, such as gastric cancer.
- It is commonly employed in research on various digestive tract tumors.
RELATED PRODUCTS