PASM-Masson Staining: Enhancing Pathological Diagnosis Accuracy

By admin
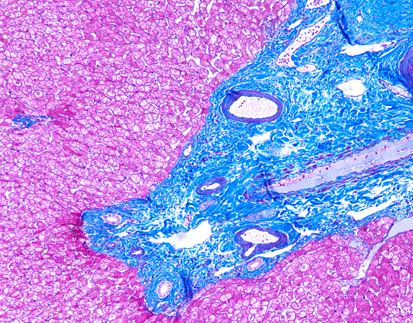
PASM-Masson staining is a crucial histological technique utilized in pathological diagnosis that highlights specific tissue components. This staining method applies the combination of periodic acid-Schiff (PAS) and Masson’s trichrome, aiding in the differentiation of various cellular structures. By staining polysaccharides, connective tissues, and collagen fibers distinctly, PASM-Masson provides clarity to the microscopic evaluation of tissues. Its ability to discern fine details contributes significantly to accurately interpreting complex pathological conditions.
Key Benefits for Pathologists
The integration of PASM-Masson staining in pathological investigations offers several advantages to pathologists. One primary benefit is its enhanced visual distinction between different tissue types, enabling pathologists to make informed diagnostic decisions. Additionally, the dual approach of PAS and Masson staining facilitates the identification of structural abnormalities and pathological changes in tissues. The comprehensive depiction of extracellular matrix components and fibrous proteins through PASM-Masson staining leads to improved diagnostic accuracy and reliability, ultimately contributing to effective patient management.
Application of PASM-Masson Staining in Renal Pathology
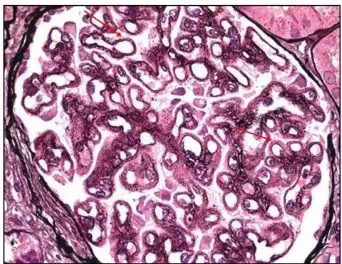
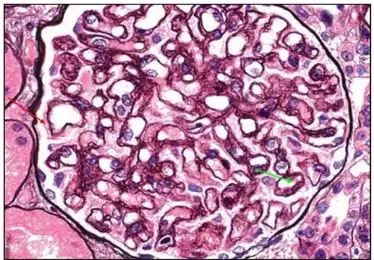
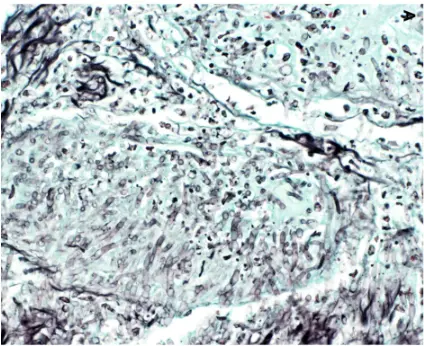
PASM-Masson staining plays a pivotal role in renal pathology by specifically visualizing the glomerular basement membrane and extracellular matrix. This capability allows for precise assessment of renal tissue architecture, particularly the delicate structures essential for kidney function. The staining method helps to emphasize the basement membrane’s presence and state so pathologists can identify any irregularities that could affect filtration processes effectively and accurately visualizing these structures assists, in spotting diseases and determining the right treatment approaches.
Identification of Basement Membrane Alterations
Changes in the basement membrane may suggest kidney issues and the use of PAS Masson staining is effective in spotting these alterations.PATHOLOGISTS depend on this method for recognizing thickening or thinning as well as breakages in the basement membrane that can signal problems, like glomerulonephritis or diabetic kidney disease. By pointing out structural deviations, PASM-Masson staining contributes to an accurate differential diagnosis and can inform prognostic evaluations.
Observing Bowman’s Capsule, Arterial Elastic Lamina, and Argophilic Protein Deposits
In addition to the basement membrane, PASM-Masson staining facilitates the observation of Bowman’s capsule, the arterial elastic lamina, and argophilic protein deposits. While assessing renal pathology, identifying these features is essential for comprehensive analysis. The method effectively stains these components, enabling pathologists to identify pathological changes within the renal vasculature and surrounding structures. This observation assists in diagnosing various renal diseases and alerts clinicians to potential complications in kidney health.
Specific Renal Conditions Identified by PASM-Masson Staining
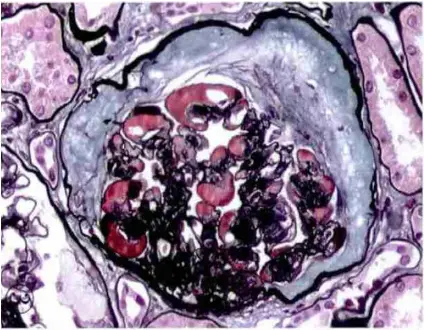
Membranous Glomerulonephritis Characteristics: Stage 2 and Stage 3
PASM-Masson staining is instrumental in identifying the key characteristics of membranous glomerulonephritis, particularly in stages 2 and 3. These stages are marked by distinct structural changes in the glomeruli, which can be effectively highlighted through this staining method. The visualization of immune complex deposits and thickened glomerular membranes allows pathologists to accurately classify the disease stage and devise appropriate management strategies. As such, PASM-Masson staining serves a critical role in not only identifying but also monitoring the progression of membranous glomerulonephritis.
Diabetic Nephropathy: Mesangial Nodule Sclerosis and Protein Deposits
In the context of diabetic nephropathy, PASM-Masson staining aids in recognizing mesangial nodule sclerosis and the presence of protein deposits within the renal structure. The technique emphasizes alterations in the mesangial region, helping pathologists distinguish between different stages of diabetic nephropathy. By highlighting specific protein deposits, PASM-Masson staining provides insights into the pathological processes involved in diabetes-related kidney impairment, allowing for timely and targeted therapeutic interventions.
Application of PASM-Masson in Fungal Staining
Mechanism of Hexamine Silver Staining for Fungi
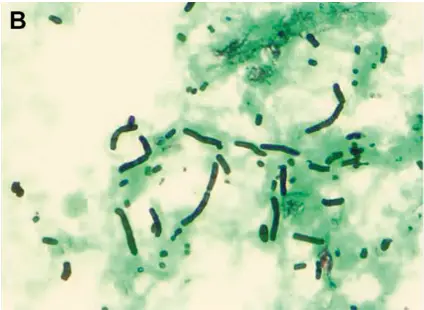
PASM-Masson staining also extends its utility to fungal staining, particularly through hexamine silver staining. This technique is very effective at pointing out organisms in tissue samples by specifically coloring the polysaccharides present in fungal cell walls. It helps to distinguish infections that might go unnoticed due to the improved contrast provided by hexamine silver staining. This clarification is essential in tissue biopsies where it’s important to identify the cause, for proper treatment.
Identifying Different Types of Fungal Infections
Mucormycosis
Mucormycosis is a severe fungal infection that can be effectively diagnosed using PASM-Masson staining. By visualizing the unique hyphal structure associated with this infection, pathologists can swiftly identify its presence in tissue samples. This level of accuracy is vital in ensuring quick medical interventions, as mucormycosis poses significant risks to patient health.
Aspergillosis
In the same way as PASM Masson staining helps detect Aspergillosis. A significant fungal infection. The unique morphological characteristics of Aspergillosis species can be seen through this staining technique. This early and precise detection, via PASM Masson staining can enhance treatment choices. Ultimately benefit patient results.
Histoplasmosis
Histoplasmosis diagnosis also benefits from the use of PASM-Masson staining. The ability to distinguish Histoplasma capsulatum within tissue sections enhances the diagnosis of this systemic fungal infection. By clearly outlining the presence of fungal elements in tissues, PASM-Masson staining enables pathologists to confirm histoplasmosis efficiently and accurately.
Solutions
For those seeking further diagnostic solutions, Celnovte’s Solutions offers a range of innovative tools tailored for enhancing pathological diagnostics. Integrating advanced techniques like PASM-Masson staining into your laboratory practice can elevate the accuracy of your diagnostic results.
Use in Other Pathogen Detection
Non-fungal Pathogens Detected by PASM-Masson Staining
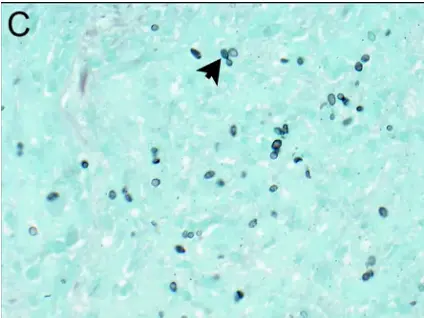
PASM-Masson staining extends its applications beyond fungal pathogens, offering valuable insights for the identification of non-fungal infections as well. Its utility encompasses the detection of various bacterial agents and parasitic infections, which can significantly influence treatment decisions. The dual capabilities of PASM-Masson allow pathologists not only to visualize typical cellular structures but also to unveil pathological alterations linked to non-fungal pathogens. This multifaceted approach enhances the overall diagnostic accuracy, facilitating timely and effective patient management.
Acid-Fast Bacilli and Non-acid-fast Bacteria
One notable advantage of employing PASM-Masson staining is its effectiveness in identifying acid-fast bacilli, particularly in cases of tuberculosis. The unique staining characteristics confer clarity when distinguishing these bacteria within tissue sections, which can be crucial in confirming an infection. Additionally, PASM-Masson staining can highlight non-acid-fast bacteria, which are equally significant in various infectious processes. The ability to highlight these distinct pathogens supports a comprehensive assessment of pathological conditions, allowing for more tailored therapeutic regimens.
Parasitic Infections and Viral Infected Cells
PASM-Masson staining also proves beneficial in the detection of parasitic infections. For example, various protozoan and helminthic infections can be visualized effectively through this staining method. Moreover, the technique offers the means to observe virally infected cells, elucidating key morphological changes that occur in response to viral infections. Pathologists can utilize PASM-Masson staining to identify these infections, thereby improving diagnostic workflows and enhancing treatment strategies.
Example: Bacillus Anthracis Detection in Bronchoalveolar Lavage Specimens
The detection of Bacillus anthracis, the causative agent of anthrax, serves as a pertinent example of how PASM-Masson staining can be effectively utilized. When analyzing bronchoalveolar lavage specimens, pathologists can identify the presence of these bacteria through the distinctive morphological patterns that emerge with PASM-Masson staining. This early identification is essential, as it underscores the necessity for prompt medical interventions, thereby averting potential complications associated with anthrax.
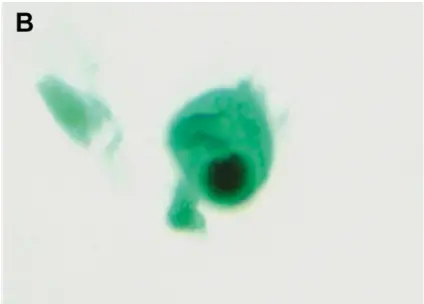
Example: Cytomegalovirus Inclusion Bodies with “Owl’s Eye” Appearance
Another significant application of PASM-Masson staining is in the detection of cytomegalovirus (CMV) inclusion bodies. The distinct “owl’s eye” appearance seen within infected cells can be effectively highlighted using this staining method. This capability is critical for pathologists aiming to detect CMV-associated tissue involvement, which may encompass severe manifestations in immunocompromised patients. The visualization provided by PASM-Masson staining facilitates accurate diagnosis and guides appropriate management options.
Common Challenges in Manual PASM-Masson Staining Procedures
Dye Usage and Timing Control to Avoid Uneven or Deep Background Stains
One of the primary challenges encountered in the manual execution of PASM-Masson staining lies in achieving consistent dye usage and precise timing. Inadequate control over dye application can lead to uneven staining patterns or overly deep background stains, complicating the overall interpretation of results. Pathologists must ensure that the staining process is meticulously timed to achieve optimal differentiation between structures without overwhelming background discoloration. By adhering to protocols and monitoring timing closely, it is possible to enhance the overall effectiveness of the staining procedure.
Precise Re-staining Process to Highlight Target Structures Without Over-staining Background
Re-staining is a critical step in the PASM-Masson staining protocol, however, it can present challenges if not executed correctly. This process requires a delicate balance to ensure that target structures are effectively highlighted while preventing excessive background staining. Pathologists should be well-versed in their techniques to avoid pitfalls that can obscure diagnostic clarity. Practicing precision in the re-staining process allows for greater accuracy in identifying critical structures within the tissue samples.
Temperature Management During Specific Steps to Ensure Dye Effectiveness
Temperature plays a significant role in the effectiveness of PASM-Masson staining. Maintaining optimal temperatures throughout the staining process is essential to preserve dye integrity and reactivity. Temperature variations can lead to altered staining reactions, potentially compromising the results. Therefore, it is imperative to monitor and regulate temperatures closely during critical steps, ensuring that the staining procedure yields reliable and accurate outcomes.
Preventing Contamination When Adding Samples to Dye Solution to Maintain Results Authenticity
Contamination is a potential risk during the PASM-Masson staining process, particularly when adding samples to the dye solution. To ensure the authenticity of results remains intact and accurate during pathological assessments pathologists need to follow strict protocols to prevent any form of cross-contamination or introduction of foreign substances. This involves cleaning the instruments and containers utilized throughout the staining process to minimize contamination risks and uphold result integrity. By adhering to these guidelines pathologists can guarantee reliable and precise pathological evaluations.
Proper Differentiation and Washing Steps to Eliminate Excess Dye
Proper differentiation and thorough washing procedures are crucial components in securing optimal PASM-Masson staining results. Excess dye can obscure critical details, hindering accurate interpretation. Pathologists need to execute washing steps with diligence to remove any superfluous dye. By applying effective differentiation techniques, the clarity of the stained sample is enhanced, leading to improved diagnostic accuracy.
Ensuring Complete Drying Before Mounting Slides to Avoid Air Bubbles or Slide Displacement
Complete drying of stained slides before mounting is another essential step in the manual PASM-Masson staining procedure. Insufficient drying can result in air bubbles or displacement of the slide, both of which can compromise the quality of the microscopic evaluation. Pathologists should prioritize thorough drying practices to ensure that the stained sections remain intact and accurately positioned. By addressing this challenge, the overall reliability of the diagnostic results can be significantly strengthened.
Solutions
For those seeking further diagnostic solutions, integrating comprehensive techniques like Celnovte Solutions into your laboratory practice can enhance the accuracy and reliability of pathogen detections. Employing innovative and strategic methods such as PASM-Masson staining will contribute to improved diagnostic capabilities and patient outcomes in complex pathological evaluations.
RELATED PRODUCTS