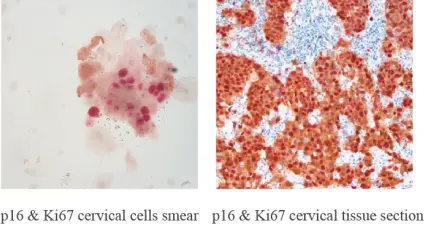
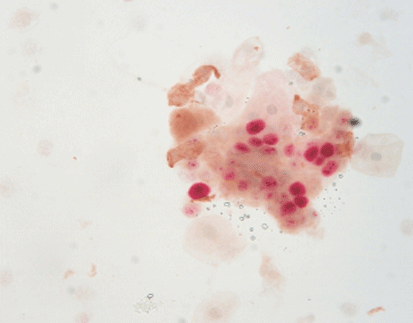
P16/Ki-67 Dual Staining: A Powerful Tool for Cervical Cancer Screening and Diagnosis
2024-11-19
By admin
Detecting cancer early is crucial for better treatment outcomes and is a major issue in global health. The P16/Ki-67 dual staining technique has emerged as a powerful tool in this fight, offering valuable insights into the cellular processes underlying cervical cancer development.
What is P16/Ki-67 Dual Staining?
P16/Ki-67 dual staining an immunohistochemical method is utilized to identify two distinct biomarkers— P16 and Ki67— within a single tissue sample enabling a thorough examination of cellular alterations linked to cervical cancer.
Understanding the Biomarkers
p16 (INK4a): This protein that suppresses tumors is crucial in regulating cell cycles to prevent cell growth tendencies in precancerous and cancerous cervical lesions where there is an abnormality, in controlling normal cell cycles.
Ki-67: This specific protein indicates cell growth. It is found in cells that are actively dividing. A high level of Ki-67 expression signals a pace of cell multiplication and may point to the presence of cancerous developments.
How it Works
The p16/Ki-67 staining technique is performed by using particular antibodies that attach to p16 and Ki-67 proteins in a tissue sample simultaneously visualizing both markers with different colored dyes. The patterns of staining give insights into cellular activities, in action.
Advantages of P16/Ki-67 Dual Staining
Using P16/Ki-67 dual staining provides benefits compared to using single staining techniques, in cervical cancer screening and diagnosis.
Enhanced Sensitivity and Specificity: The technique improves the accuracy of detecting irregularities by recognizing two distinct biomarkers simultaneously. This combined strategy reduces the occurrence of positive and negative outcomes resulting in more dependable diagnoses.
Improved Risk Stratification: The combined detection of p16 and Ki-67 offers an evaluation of cervical lesion seriousness aiding healthcare providers in deciding on tailored treatment plans, for individual patients.
Early Detection of High-Grade Lesions: The examination of both p16 and Ki-67 expression is highly beneficial for pinpointing grade cervical intraepithelial neoplasia (CIN) which is a pre-cancerous state with an elevated likelihood of advancing to cervical cancer development later on, in life. When high-grade CIN is detected and treated early it can greatly lower the chances of developing cervical cancer.
Clinical Applications of P16/Ki-67 Dual Staining
P16/Ki-67 dual staining has found widespread clinical application in the management of cervical cancer.
Screening and Diagnosis: The method is now commonly employed alongside the Pap smear examination in programs, for checking for cervical cancer to pinpoint women with a greater likelihood of developing the disease. Especially those who have unusual Pap smear outcomes or positive HPV test results.
Triage of HPV-Positive Women: High-risk HPV-positive women can benefit from using p16/Ki-67 staining to pinpoint those at a greater risk of precancerous or cancerous growths, in their bodies This method aids in prioritizing patients for additional scrutiny and care measures.
Guiding Treatment Decisions: The findings from using p16/Ki-67 staining can help doctors make well-informed choices regarding tailored treatment plans, for each patient’s needs This could involve careful observation or more regular checkups and procedures to address pre-cancerous conditions.
Monitoring Treatment Response: In situations p16/Ki-67 dual staining can be utilized to assess how well treatment is working for cervical precancerous lesions. Test results showing expression of both biomarkers could indicate a good response, to treatment.
Celnovte: A Leading Provider of P16/Ki-67 Dual Staining Solutions
Celnovte Biotech is a leading provider of high-quality immunohistochemistry products, including P16/Ki-67 dual staining kits and reagents. The company’s dedication, to innovation and excellence, has led to the creation of an array of solutions tailored to fulfill the requirements of healthcare facilities and scientific organizations.
Key Features of Celnovte’s P16/Ki-67 Dual Staining Kit:
Fixed with spray: This feature allows for convenient and easy-to-use preparation of the staining solution.
MicroStacker detection system: The kit incorporates Celnovte’s innovative MicroStacker™ technology, ensuring enhanced sensitivity and specificity in detecting both p16 and Ki-67 biomarkers.
Ready-to-use reagents: The set comes with everything you need for the staining process to make it easier and reduce the chances of mistakes. However, focusing on Celnovte’s products with ready-to-use reagents and our previous talks, about Celnovte providing such reagents suggests that the p16/Ki-67 dual staining kit probably has this convenience as well. Independent verification of this information may be necessary.
Excellent staining results: Celnovte’s p16/Ki-67 dual staining kit produces high-quality staining results, making interpretation clear and straightforward.
Conclusion
The combination of p16/Ki-67 staining marks notable progress in screening and diagnosing cervical cancer. This technique allows for detecting two biomarkers simultaneously which gives insights into cellular transformations linked to cervical cancer progression. With its capability to identify cancer at a stage accurately assess risks and guide treatment choices p16/Ki-67 dual staining is significantly enhancing outcomes, for women facing cervical cancer.
FAQs
- How is p16/Ki-67 dual staining interpreted?
When both p16 and Ki-67 are expressed together in a cell or group of cells and cytoplasm colors in yellow while the nucleus is reddish brown; it signifies a positive outcome. The analysis should consider characteristics and clinical background, for accurate interpretation.
- Can p16/Ki-67 dual staining be used for other types of cancer?
p16/Ki-67 dual staining typically is used for the detection and diagnosis of cancer, but investigators study its use for other types, such as head and neck squamous cell carcinomas and anal cancers.
- The limitations of p16/Ki-67 dual staining
Specialized tools and skilled professionals are essential, for analysis using this method. The possibility of positives or negatives emphasizes the importance of considering the clinical context carefully. This method should not be solely relied upon for diagnosis; it is best used alongside clinical and pathological data.
- More information about p16/Ki-67 dual staining
Your healthcare provider may have more detailed information about P16/Ki-67 dual staining and its role in cervical cancer screening and diagnosis. Additionally, some reputable medical websites, such as websites from professional medical societies, do provide state–of–the-art information.