Mastering the EBER Probe for Accurate EBV Identification in Cells
 2024-08-29
2024-08-29
By admin
 The EBER Test for EBV Detection
The EBER Test for EBV Detection
What is the EBER Test
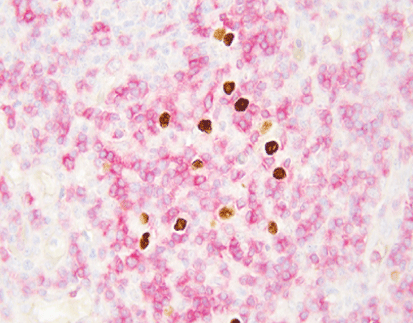
The EBER test, which stands for Epstein-Barr encoding small RNAs, is a molecular diagnostic assay designed to detect the presence of Epstein-Barr virus (EBV) within tissue samples. Primarily, it identifies EBER1 and EBER2, which are non-coding RNAs synthesized by the virus during its latent phase. The primary purpose of the EBER test is to establish a definitive diagnosis of EBV infection, particularly in cases where the virus is implicated in malignancies. This test serves as a vital tool for clinicians to classify and manage EBV-associated diseases accurately.
Importance of Accurate EBV Identification
Accurate identification of EBV is crucial, as this virus has been linked to various lymphoproliferative disorders and malignancies, including Burkitt lymphoma and nasopharyngeal carcinoma. Misdiagnosing EBV-related conditions can lead to inappropriate therapeutic interventions and poor clinical outcomes. Consequently, the EBER test’s reliability plays an integral role in the overall diagnostic process, ensuring that clinicians can implement targeted treatments based on precise data. Furthermore, accurate identification aids in the understanding of EBV’s role in disease pathogenesis, which has implications for further research and therapeutic advancements.
Role of EBER Probe in EBV Detection
The EBER probe is a pivotal component of the EBER test, acting as a specific hybridization tool to detect latent EBV within cells. This probe facilitates the visualization of EBER RNA through in situ hybridization techniques. The specificity of the EBER probe ensures that clinicians can discern between EBV-positive and negative cells, providing an accurate assessment of viral presence in biopsied tissues. In essence, the EBER probe enhances the sensitivity and specificity of the diagnostic process, positioning it as a cornerstone of EBV detection methodologies.
Techniques Utilized in EBV Identification
Overview of In Situ Hybridization (ISH)
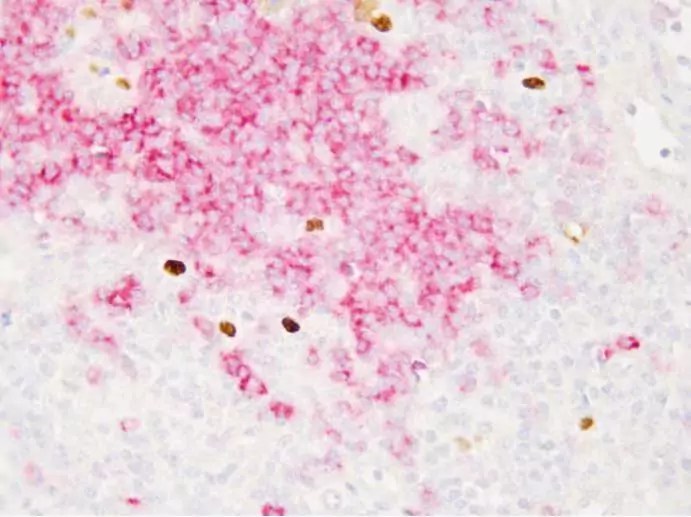
In situ hybridization (ISH) is a molecular technique leveraged for the detection of specific nucleic acid sequences within fixed tissues or cells. This approach often employs labeled probes that hybridize to target sequences, enabling visualization under a microscope. In the context of EBV detection, ISH utilizes the EBER probe to directly identify the presence of EBV RNA in tissue samples. The sensitivity of ISH makes it an invaluable tool for diagnosing EBV-associated malignancies, as it allows pathologists to observe the localization and expression patterns of the viral RNAs in the morphological context of the tissue.
Methodology of ISH Using Commercial Probes
Diagnostic laboratories typically utilize commercial EBER probes optimized for sensitivity and specificity. The procedure involves preparing tissue sections, followed by deparaffinization and hydration. Subsequently, the sections are treated to enhance the binding affinity between the probe and the target RNA. Hybridization occurs in a controlled environment to minimize non-specific binding, and following washing steps, the bound probes are visualized through various detection systems. This standardized methodology not only facilitates accurate EBV detection but also contributes to consistency across different clinical settings.
Comparative Accuracy of Different Probes
The accuracy of EBV identification can vary depending on the probes used in ISH. Comparative analyses have shown that commercially available EBER probes demonstrate heightened sensitivity compared to other detection methods, such as PCR. These developments underline the importance of using optimized probes for effective clinical diagnostics. Additionally, the choice of probe can influence the outcome of the test, making it vital to select probes validated through rigorous scientific studies. Understanding the comparative advantages of various probes can enhance clinicians’ ability to select appropriate diagnostic tools for specific patient populations.
Applications of EBER Probe Across Various Clinical Scenarios
Diagnosing EBV-Associated Cancers
The applications of the EBER probe extend significantly into the realm of oncology, particularly in diagnosing EBV-associated malignancies. The test can identify the presence of EBV in tumors, aiding in the classification and treatment planning for specific cancers.
Case of Non-Small Cell Lung Cancer
In cases of non-small cell lung cancer (NSCLC), the detection of EBER may reveal the presence of EBV in tumor specimens. This association has implications for prognosis and treatment, as EBV-positive NSCLC may respond differently to therapies. Using the EBER probe, clinicians can obtain vital information that can influence treatment approaches and clinical management pathways, ensuring a tailored approach to patient care.
Relevance to Lymphoma Diagnosis and Monitoring
The EBER probe is also particularly relevant in the context of lymphoma diagnosis and monitoring. Certain types of lymphomas, including Hodgkin lymphoma and various non-Hodgkin lymphomas, are closely associated with EBV infection. Early detection of EBV within lymphoma tissue can assist in prognosis and inform follow-up strategies. Moreover, monitoring EBV expression through the EBER probe can provide insights into treatment efficacy, recurrence risk, and overall patient outcomes, thus delivering a comprehensive framework for managing these complex malignancies.
Detecting EBV in General Patient Populations
Beyond specialized cancer diagnoses, the EBER probe is beneficial in monitoring EBV within general patient populations.
Analysis Techniques Using PCR and Viral Load Assessment
When assessing EBV in the broader population, molecular techniques such as PCR are often employed alongside the EBER test. These methodologies can quantify viral load and identify active infections, contributing to an improved understanding of EBV’s epidemiology. The integration of EBER test results with viral load assessments enhances the diagnostic algorithm, offering a multidimensional perspective on EBV status in patients. Understanding these dynamics is essential in managing infectious diseases associated with EBV, including infectious mononucleosis and post-transplant lymphoproliferative disorders.
Advancements in EBER Probe Utilization
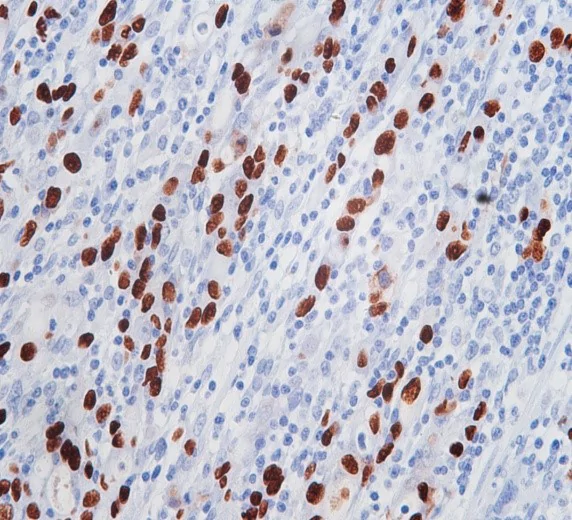
Celnovte’s Construction
Celnovte has pioneered a robust foundation for the production of high-performance EBER probes that cater to the diagnostic needs of EBV research and clinical practice. Their advanced manufacturing processes incorporate a specialized design that maximizes the binding affinity of the probe to target RNA sequences, leading to improved detection rates. Furthermore, Celnovte prioritizes rigorous quality control measures throughout the construction process, ensuring that each probe batch meets the stringent standards necessary for medical diagnostics. This focus on precision and reliability positions Celnovte at the forefront of EBER probe development, making their probes highly sought after in the context of EBV detection.
EBER/IHC Dual Staining Kit from Celnovte
Celnovte’s EBER/IHC Dual Staining Kit offers a unique approach to diagnosing lymphoma by combining two powerful techniques: in situ hybridization (ISH) using an EBER probe and immunohistochemistry (IHC). The kit comes in two variations, specifically targeting and identifying T or B lymphocytes within the context of Epstein-Barr virus (EBV) infection.
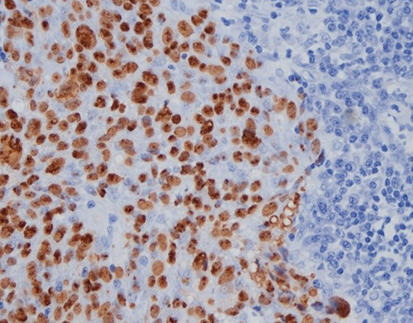
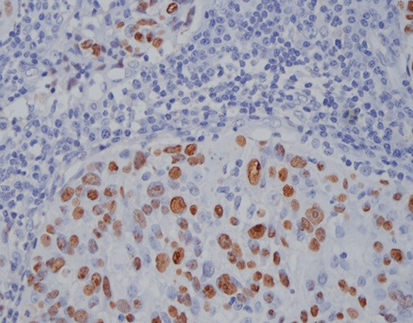
The EBER Probe (ISH) component of the kit is designed to detect the presence of EBV in situ, which is crucial in diagnosing certain types of lymphoma associated with this virus. By combining this with immunofluorescence staining for cell line markers, the kit enables the visualization of the positive cell type infected with the virus. In the first variation, the EBER Probe is paired with CD3 (IHC), a marker commonly used to label T lymphocytes. This combination allows for the identification of T lymphocytes infected with EBV, which can be indicative of anaplastic T-cell lymphoma.
The second variation of the kit pairs the EBER Probe (ISH) with CD20 (IHC), a marker used to label B lymphocytes. This combination is particularly useful in the diagnosis of diffuse large B cell lymphoma, as it enables the simultaneous detection of EBV infection and the presence of B lymphocytes in the same tissue section. By providing this dual staining capability, the kit offers a comprehensive assessment of lymphoma at both the molecular and protein levels, ultimately leading to improved diagnostic accuracy and a more informed treatment plan.
EBER Probe (Automated) from Celnovte
The EBER Probe (Automated), with a code of CF6002, is a specialized diagnostic tool designed for automated use in detecting Epstein-Barr virus (EBV) infection in situ. It comes in varying specifications of 25T, 50T, and 100T, allowing for flexibility in processing samples. The automated staining process simplifies and streamlines the detection process, making it more efficient and consistent. The kit’s main components include the EBER Probe itself, along with a DAB Enhancer for enhanced staining, an Anti-digoxin Antibody for specific targeting, an EBER Positive Control for validation of results, a Pepsin Solution for tissue pretreatment, and a MicroStackerTM for efficient handling and organization of samples during the staining process. This comprehensive set of components ensures accurate and reliable detection of EBV-infected cells, facilitating accurate diagnosis and treatment planning.
Future Prospects in EBV Research with EBER Probe
The future trajectory of EBV research looks promising with continued advancements in the design and application of the EBER probe.
Integration with Next-Generation Sequencing
Integration of EBER probe findings with next-generation sequencing (NGS) technologies heralds a new era of EBV research. By combining the specificity of EBER detection with the comprehensive data generated by NGS, researchers can explore the full spectrum of EBV mutations and gene expressions within malignancies. This synergistic approach allows for a deeper understanding of how viral strains may influence disease progression and therapeutic responses. Additionally, the potential to identify novel biomarkers associated with EBV through comprehensive genomic analysis holds significant promise for future diagnostics and targeted treatments.
Expanding Applications Beyond Oncology
There is growing interest in expanding the applications of the EBER probe beyond oncology and diagnosing EBV-related malignancies. Emerging studies are investigating the role of EBV in autoimmune diseases, neuroinfections, and chronic fatigue syndrome, thus posing important questions regarding the virus’s broader impact on health. The EBER probe’s versatility allows for its application in various tissue types and cell populations, potentially facilitating more extensive research into the virus’s influence in different contexts. As scientists continue to investigate these avenues, the EBER probe’s capacity for precise identification and characterization may yield valuable insights that inform clinical practice across diverse disciplines.
Conclusion
In summary, the advancements provided by EBER probe technology, particularly those developed by companies like Celnovte, significantly enhance the capacity for accurate EBV detection across various clinical scenarios. The combination of innovative construction methods, automated systems, and dual-staining capabilities has paved the way for improved diagnostic pathways. Looking forward, the application of EBER probes in conjunction with cutting-edge research methodologies sets the stage for significant discoveries in the understanding and management of EBV-related diseases. The future of EBV research is bright, with EBER probes poised to continue facilitating influential findings that resonate throughout the fields of oncology, virology, and beyond.
RELATED PRODUCTS