How to Identify and Target Claudin18.2: A Definitive Guide
2024-05-02
By admin
Understanding Claudin18.2 and Its Role in Cancer
Introduction to Claudin18.2
Claudin18.2 (CLDN18.2) is a transmembrane protein that plays a crucial role in the development and progression of various primary malignant tumors, including gastric cancer (GC) and esophago-gastric junction adenocarcinomas (EGJA). Structurally, Claudin18.2 is localized on the apical side of the cell membrane and has extracellular loops capable of binding monoclonal antibodies.
The Structure and Function of Claudin18.2
Claudin18.2 is a highly selective biomarker with frequent abnormal expression during the occurrence and development of various primary malignant tumors, particularly in gastrointestinal malignancies. It participates in the proliferation, differentiation, and migration of tumor cells, making it an effective molecular target for potential anti-tumor drugs.
Role of Claudin18.2 in Tumor Progression
Recent scientific investigations have pinpointed CLDN18.2 as a pivotal target for the therapeutic intervention in gastric cancer, attributable to its selective expression within tumor cells and its crucial involvement in tumor progression. Research indicates that Claudin18.2 is implicated in various signaling pathways associated with tumorigenesis and may function either as an oncogene or a tumor suppressor gene across different types of tumors.
Why Target Claudin18.2?
Selective Expression in Cancer Cells
Approximately 33% to 37% of patients with solid tumors exhibit high expression of CLDN18.2, with positive expression being significantly associated with shorter overall survival in gastric cancer patients.
The Potential for Improved Treatment Outcomes
Positive Claudin18.2 expression levels are associated with the degree of tumour differentiation, Borrmann type, PD-L1 expression levels, and survival rates among GC patients. This suggests that targeting CLDN18.2 presents an opportunity for improved treatment outcomes by addressing specific characteristics associated with this protein’s expression.
The Significance of Identifying Claudin18.2 in Patients
The identification of CLDN18.2 in patients is of paramount importance in the realm of cancer diagnosis and treatment. By understanding the methods for detecting Claudin18.2 expression and its impact on patient care, healthcare professionals can personalize treatment strategies and predict treatment efficacy.
Methods for Detecting Claudin18.2 Expression
The detection of Claudin18.2 expression is crucial for accurate diagnosis and targeted therapy in patients with various malignancies, particularly gastric cancer (GC) and gastroesophageal junction adenocarcinomas (GEJ). Healthcare providers rely on advanced diagnostic techniques to identify the presence of CLDN18.2 in tumor cells, enabling them to tailor treatment plans according to the specific molecular characteristics of each patient’s cancer.
CAS PubMed Google Scholar Resources: Clinicians frequently utilize resources such as CAS, PubMed, and Google Scholar to access the latest research studies and clinical findings related to Claudin18.2 expression in different types of cancer. These platforms provide a wealth of information on the role of Claudin18.2 as a therapeutic target and its association with clinicopathological features, offering valuable insights into its significance in cancer management.
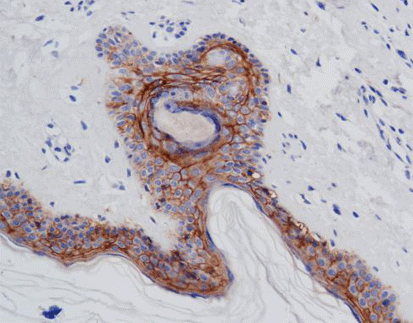
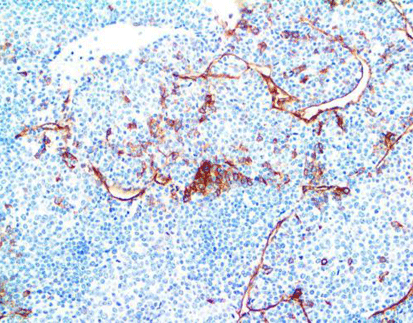
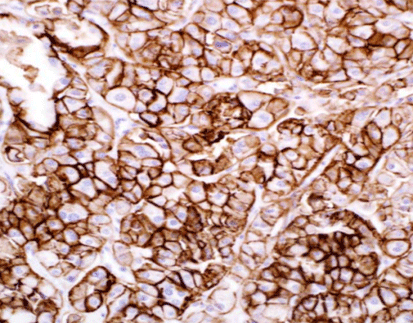
Advanced Diagnostic Techniques: Immunohistochemistry (IHC) plays a pivotal role in detecting CLDN18.2 expression levels within tumor tissues, allowing pathologists to assess the extent of protein expression and its correlation with clinical outcomes. Additionally, emerging technologies such as liquid biopsy hold promise for non-invasive assessment of CLDN18.2 status, providing a minimally invasive approach to monitor disease progression and treatment response.
The Impact of Claudin18.2 on Patient Care
The presence of Claudin18.2 has far-reaching implications for patient care, influencing personalized cancer treatment approaches and serving as a prognostic indicator for treatment efficacy.
Personalizing Cancer Treatment: Studies have shown that CLDN18.2 serves as an independent prognostic factor for patients with GC, indicating its potential as a therapeutic target for tailored interventions based on individual tumor characteristics.
Predicting Treatment Efficacy: The expression levels of CLDN18.2 have been linked to immune infiltrating cells and PD-L1 expression in gastric tumors, shedding light on their predictive value for treatment response and overall prognosis among patients with digestive malignancies.
By leveraging these insights into the significance of identifying CLDN18.2 in patients, healthcare providers can optimize treatment strategies and improve clinical outcomes through targeted therapies tailored to each patient’s unique molecular profile.
Advances in Claudin18.2 Targeted Therapies
In recent years, substantial progress has been achieved in the development of targeted therapies focused on Claudin18.2 (CLDN18.2), presenting promising opportunities for the treatment of various malignancies, especially gastric cancer and esophago-gastric junction adenocarcinomas.
The Development of Claudin 18.2 Antibodies
Monoclonal Antibodies and Their Mechanism
Monoclonal antibodies targeting Claudin18.2 have emerged as a focal point in precision medicine for cancer therapy. These antibodies are designed to specifically recognize and bind to the Claudin18.2 protein with high affinity, thereby exerting their therapeutic effects through multiple mechanisms.
Zolbetuximab is a prime example of a monoclonal antibody developed to target CLDN18.2 in solid tumors, including gastric cancer and pancreatic ductal adenocarcinoma. Clinical studies have demonstrated its specificity and antitumor potency, showcasing its ability to induce effective and target-selective antibody-dependent cell-mediated cytotoxicity (ADCC) against tumor cells expressing CLDN18.2.
Zolbetuximab: A Case Study
Clinical trials evaluating zolbetuximab in Claudin18.2-positive tumors have yielded encouraging results, underscoring its potential as an effective therapeutic intervention for patients with advanced unresectable or metastatic gastric or gastroesophageal junction adenocarcinoma.
For instance, a phase II study investigating zolbetuximab combined with epirubicin, oxaliplatin, and capecitabine (EOX) demonstrated a notable objective response rate (ORR) of 39.0% compared to 25.0% with EOX alone among CLDN18.2-positive patients with advanced gastric or gastroesophageal junction cancer in the first-line setting.
Moreover, zolbetuximab achieved satisfactory outcomes when used in combination with IL-2 and zoledronic acid, leading to disease control in a substantial proportion of evaluable patients, including partial responses and stable disease states.
Emerging Therapies and Clinical Trials
CAR T-Cell Therapies Targeting Claudin18.2
The emergence of chimeric antigen receptor (CAR) T-cell therapies targeting Claudin18.2 represents a groundbreaking approach in cancer immunotherapy. By genetically modifying T-cells to express CARs specific to CLDN18.2, these innovative therapies hold immense potential for precise targeting of tumor cells showing high expression levels of this protein.
Preliminary data from preclinical studies has shown promising results regarding the safety and efficacy of CAR T-cell therapies directed against CLDN18.2-expressing tumor cells across various malignancies.
As clinical trials continue to evaluate the therapeutic benefits of these novel interventions, ongoing research endeavors aim to elucidate the full spectrum of their antitumor activity and their potential role in improving treatment outcomes for patients with advanced solid tumors characterized by heightened CLDN18.2 expression.
By leveraging these cutting-edge developments in targeted therapies focused on Claudin18.2, clinicians are poised to revolutionize cancer treatment paradigms by harnessing the specificity and potency of these interventions tailored to address the unique molecular profiles of individual patients’ tumors.
The Future of Claudin18.2 Research and Treatment
As research on CLDN18.2 continues to advance, ongoing studies and extended data play a pivotal role in shaping the future of cancer treatment strategies. These endeavors aim to explore the next frontier in Claudin18.2 research while addressing the challenges and opportunities associated with targeting this transmembrane protein.
Ongoing Studies and Extended Data
The Next Frontier in Claudin18.2 Research
Recent scientific research findings have underscored the emergence of Claudin18.2 as a promising therapeutic target for advanced solid tumors, particularly gastric cancer (GC) and pancreatic ductal adenocarcinoma (PDAC). Studies have revealed that different therapeutic agents targeting CLDN18.2 for cancer immunotherapy, including monoclonal antibodies, bispecific antibodies, CAR-T cells, and antibody-drug conjugates (ADCs), have shown encouraging preliminary results in terms of clinical efficacy.
In particular, ongoing clinical trials investigating monoclonal antibody therapy and CAR-T therapy have disclosed valuable clinical trial data demonstrating good safety profiles in Claudin18.2-positive gastric and pancreatic cancer populations. These findings highlight the potential of CLDN18.2-targeted therapies to revolutionize treatment paradigms for patients with locally advanced unresectable or metastatic gastric or pancreatic tumors.
Furthermore, identifying the optimal patient population via biomarker studies is crucial for maximizing the potential benefit of Claudin18.2-targeted therapies. Future efforts for developing these therapies should aim to maximize binding affinity for the target, minimize immunogenicity, and optimize half-life to exert antitumor activity with minimal adverse effects.
Challenges and Opportunities
While multiple Claudin18.2-targeted therapies appear promising for treating patients with CLDN18.2-positive cancers, several challenges need to be addressed in their ongoing development to ensure safe and effective treatments.
One key challenge lies in optimizing the specificity and efficacy of therapeutic agents targeting CLDN18.2 while minimizing off-target effects on healthy tissues expressing low levels of this protein. Additionally, maximizing the clinical benefits of these therapies requires comprehensive understanding of their impact on patient outcomes across various malignancies characterized by heightened Claudin18.2 expression.
Amidst these challenges lie significant opportunities to leverage innovative technologies and precision medicine approaches to advance Claudin18.2-targeted therapies further.
The Role of Technology in Advancing Treatment
Innovations in Cell Therapy
Advanced cell therapy, utilizing genetically modified T cells to express a chimeric antigen receptor (CAR), has emerged as a crucial approach in cancer treatment, particularly for solid tumors showing high expression levels of Claudin18.2.
Preliminary data from humanized anti-CLDN18.2 autologous CAR-T cell preclinical studies have demonstrated antigen-specific anti-tumor effects on GC, offering promising options for patients with advanced gastric or pancreatic tumors expressing Claudin18.2.
The Importance of Data in Personalized Medicine
The integration of comprehensive patient data into personalized medicine approaches holds immense promise for optimizing treatment outcomes among individuals with Claudin18.2-positive cancers.
By leveraging real-world evidence from extended data tables and supplementary tables derived from ongoing clinical trials, healthcare providers can gain valuable insights into the efficacy and safety profiles of Claudin18.2-targeted therapies across diverse patient populations.
These innovative strides underscore the transformative potential of technology-driven advancements in tailoring precision treatments based on individual molecular profiles within the realm of cancer care.
Conclusion
In conclusion, Claudin18.2 (CLDN18.2) emerges as a highly selective marker protein that holds immense potential for the diagnosis and treatment of various primary malignant tumors, particularly in the realm of digestive malignancies such as gastric cancer (GC), gastroesophageal junction (GEJ) cancer, esophageal cancer, and pancreatic cancer. The restricted expression of CLDN18.2 in healthy tissues and its abnormal overexpression in a range of malignancies position it as an ideal therapeutic target for precise detection, diagnosis, and treatment.
The structure and distribution of Claudin18.2 distinguish it from other proteins within the same family, such as CLDN18.1, highlighting its unique role in maintaining the barrier function of gastric mucosa while undergoing abnormal changes during the development of various malignant tumors. Its tissue-specific expression and modulation by key signaling pathways underscore its significance as a potential specific marker for targeted therapies across different tumor types.
Multiple clinical trials focusing on Claudin18.2-targeted therapies have shown promising early results, with some demonstrating potent antitumor activity through antibody-dependent cellular cytotoxicity (ADCC), complement-dependent cytotoxicity (CDC), induction of apoptosis, and inhibition of cell proliferation. These advancements pave the way for personalized treatments tailored to address the unique molecular profiles of individual patients’ tumors.
The ongoing studies and extended data derived from clinical trials play a pivotal role in shaping the future landscape of cancer treatment strategies centered around Claudin18.2 research. Challenges related to optimizing specificity and efficacy while minimizing off-target effects present opportunities to leverage innovative technologies and precision medicine approaches to advance Claudin18.2-targeted therapies further.
Celnovte Biotechnology Co., Ltd (Celnovte) is a renowned biotechnology company that specializes in the research and development, manufacturing, and distribution of pathological diagnostic reagents and instruments. With a strong focus on Immunohistochemistry and in-situ hybridization, Celnovte has made significant contributions to the field of cancer diagnostics and treatment.
One of Celnovte’s key offerings is the MicroStacker™ IHC detection system, which is known for its exceptional sensitivity and specificity. This system plays a pivotal role in detecting Claudin18.2 expression levels within tumor tissues, allowing pathologists to assess the extent of protein expression and its correlation with clinical outcomes. This aligns with the discussion in the article above about the importance of advanced diagnostic techniques in cancer management.
Moreover, Celnovte’s SuperISH™ RNA in-situ hybridization technology enables detection of RNA targets at the single molecular level and single cell resolution. This technology can provide valuable insights into the role of Claudin18.2 as a therapeutic target and its association with clinicopathological features.
In addition to their innovative diagnostic technologies, Celnovte is also leading in its innovation of fully automated instrumentation, such as IHC stainers, H&E stainer, special stainer, cytopathology instrumentation, and digital slide scanner. Since their launch in 2018, Celnovte has successfully installed over 600 units of fully automated IHC stainers globally, further solidifying their position as a leader in the field.
Headquartered in Zhengzhou, China, with subsidiaries in Shenzhen, Suzhou, and Maryland (USA), Celnovte has established an extensive domestic and international pathological network, reaching over 1500+ top hospitals in China and over 10+ countries worldwide. Their mission to elevate precision in cancer diagnostics and enrich patients’ lives through innovative products and services aligns with the ongoing efforts to optimize treatment strategies and improve clinical outcomes for patients with Claudin18.2-positive cancers, as discussed in the article above.
By leveraging their cutting-edge technologies and commitment to advancing scientific innovation, Celnovte is poised to make significant contributions to the future of Claudin18.2 research and treatment.