Exploring Molecular Diagnostics: Future Trends and Opportunities
2024-08-06
By admin
Overview of Molecular Diagnostics
Molecular diagnostics signifies a groundbreaking methodology in the medical sector, facilitating unmatched accuracy in diagnosing and managing diseases through the examination of biological markers at the molecular level. This discipline employs data from DNA, RNA, and proteins to detect diseases, predispositions, and treatment reactions. The objective is to offer clear insights and enable early intervention in disease management, thereby playing a crucial role in personalized medicine and enhancing patient outcomes.
Technological Advancements Driving Change
Next-Generation Sequencing (NGS)
Next-Generation Sequencing (NGS) has revolutionized molecular diagnostics by enabling rapid, high-throughput sequencing of entire genomes or specific genomic regions. NGS technology allows for comprehensive analysis and is invaluable for identifying genetic mutations associated with various diseases. Its application facilitates better understanding and diagnosis of complex conditions, including cancer and rare genetic disorders. The ability to sequence large amounts of DNA quickly and accurately has made NGS a cornerstone of modern molecular diagnostics, driving advancements in genomic medicine.
Polymerase Chain Reaction (PCR) Enhancements
The Polymerase Chain Reaction (PCR) remains one of the fundamental tools in molecular diagnostics. Recent enhancements in PCR technology have increased its sensitivity, accuracy, and speed. Techniques such as quantitative PCR (qPCR) and digital PCR (dPCR) allow for precise quantification of nucleic acids, which is critical for diagnosing infectious diseases, genetic abnormalities, and cancer. These improvements have broadened the scope of PCR applications, making it a versatile and indispensable tool in clinical laboratories worldwide.
Impact on Healthcare and Disease Management
Early Detection and Screening
Cancer Detection Methods
Molecular diagnostics has greatly enhanced cancer detection techniques. Methods such as chromogenic in situ hybridization and p16/Ki-67 double staining offer essential tools for early cancer diagnosis and screening. For example, chromogenic in situ hybridization, which examines staining outcomes under a light microscope to identify RNA, is utilized to diagnose EBV infections in tissue pathology. The use of these sophisticated techniques guarantees early detection, which is vital for effective cancer treatment and management.
Infectious Disease Screening
Infectious disease screening has also benefited immensely from molecular diagnostics. Technologies such as PCR and NGS allow for rapid and accurate detection of pathogens, including bacteria, viruses, and fungi. This precise detection is crucial in controlling outbreaks and managing infectious diseases effectively. The ability to detect infections early and accurately has transformed public health responses and clinical management of infectious diseases, ensuring timely intervention and treatment.
Personalized Medicine
Tailored Treatment Approaches
Personalized medicine is one of the burgeoning areas benefiting from molecular diagnostics. Understanding the genetic makeup of a patient enables healthcare providers to tailor treatment plans that are specific to the individual’s genetic profile. For example, pharmacogenomics, an application of molecular diagnostics, helps in predicting how patients will respond to drugs, thereby optimizing treatment regimens and minimizing adverse effects. Tailored treatment approaches enhance the efficacy of interventions and improve patient outcomes significantly.
Molecular diagnostics is ushering in a new era in precision medicine. With ongoing technological advancements and its growing incorporation into clinical practice, the potential to enhance patient care is significant. The capability to detect diseases at an early stage, customize treatments, and improve screening processes highlights the revolutionary impact of molecular diagnostics in contemporary healthcare.
Celnovte
Brief Introduction
Celnovte has carved a niche in the landscape of molecular diagnostics by offering cutting-edge products that cater to the evolving needs of clinical and research laboratories. With a focus on developing accurate, user-friendly, and innovative diagnostic tools, Celnovte is spearheading advancements that are pivotal for early disease detection, monitoring, and personalized treatment approaches.
Celnovte boasts a comprehensive product line comprising premium immunohistochemistry reagents, primary antibodies, and fully automated staining systems. Engineered to elevate the precision and speed of pathological assessments, these offerings are indispensable assets for laboratories and medical establishments globally, facilitating more efficient and accurate diagnoses.
Kappa/Lambda Probe Kit (ISH)
The Kappa/Lambda Probe Kit (ISH) is a diagnostic tool utilized in identifying and distinguishing between the two types of light chains, kappa (κ) and lambda (λ), found on immunoglobulins. These light chains are expressed in a specific ratio of approximately 2:1 by normal B-cells in lymph nodes, with kappa being more prevalent. Abnormalities in this ratio, indicating a restricted expression of either kappa or lambda, can signify monoclonal hyperplasia or the presence of tumors. This kit is particularly useful in diagnosing various B-cell lymphomas and multiple myeloma, such as diffuse large B-cell lymphoma (DLBCL), mantle cell lymphoma (MCL), chronic lymphocytic leukemia (CLL), small lymphocytic lymphoma (SLL), marginal zone lymphoma (MZL), and Burkitt’s lymphoma (BL). The Kappa/Lambda Probe Kit is compatible with Celnovte CNT330/330 stainers for a processing duration of 5.5 hours, as well as Leica machines, offering high sensitivity and specificity for accurate results. It boasts a wide range of applicability across tissue samples, including formalin-fixed, paraffin-embedded (FFPE) specimens. The kit is available in both manual and automated versions, with specifications of 25T, 50T, and 100T, catering to different laboratory needs and throughput requirements.
The use of in situ hybridization (ISH) provides high specificity and sensitivity, making it an indispensable tool in clinical pathology. By enabling the detection of clonality in lymphoid neoplasms, the Kappa/Lambda Probe Kit significantly aids in the discrimination between reactive and neoplastic B-cell proliferations, thus impacting patient care positively by guiding appropriate treatment strategies.
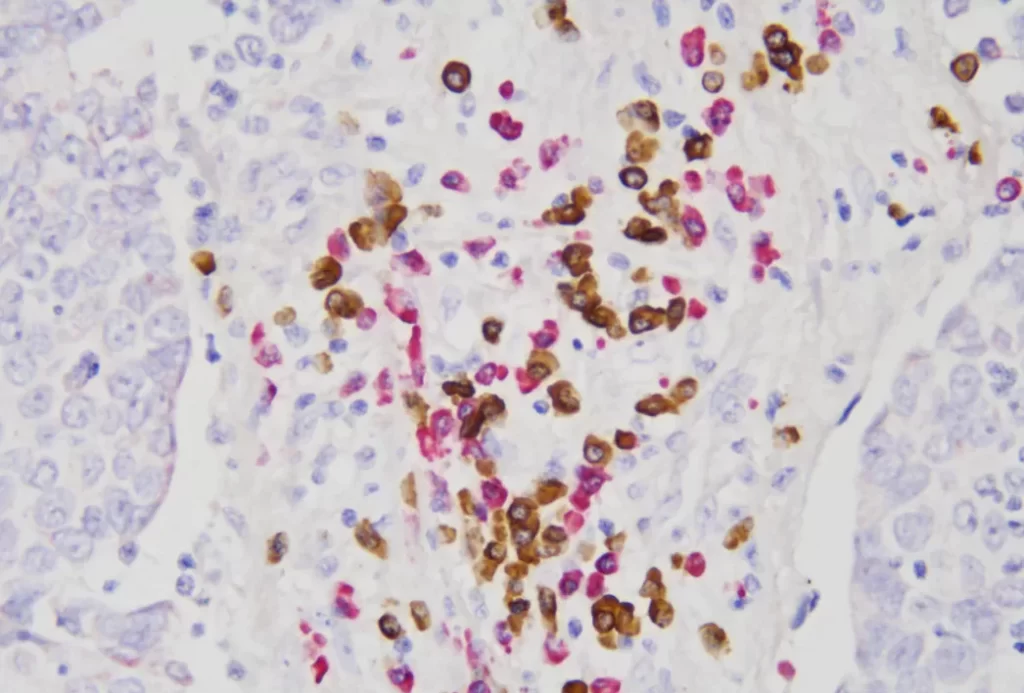
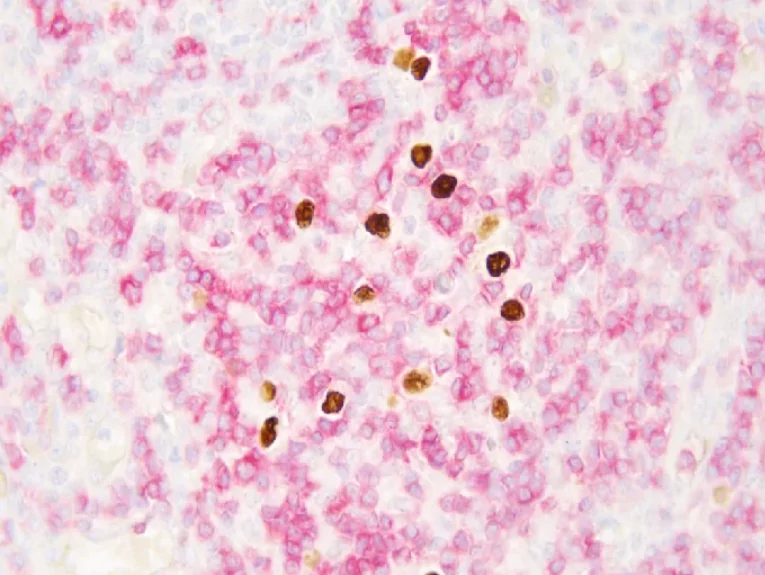
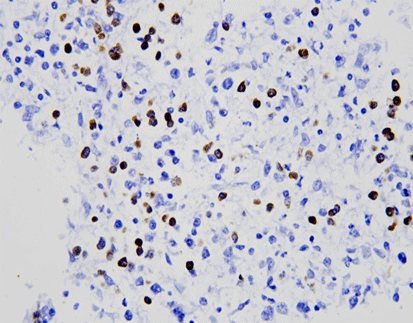
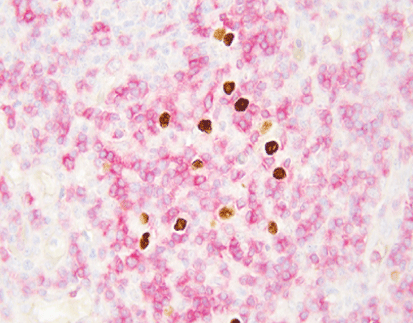
EBER/IHC Dual Staining Kit
The EBER/IHC Dual Staining Kit is a versatile diagnostic tool that combines the power of in situ hybridization (ISH) using an EBER probe to detect Epstein-Barr virus (EBV) infection with immunohistochemistry (IHC) to label specific cell line markers. This dual staining technique enables the simultaneous visualization of EBV-positive cells and their cellular identity, enhancing diagnostic precision. By pairing the EBER probe with either CD3 or CD20, the kit can distinguish between T lymphocytes (labeled by CD3) and B lymphocytes (labeled by CD20), respectively. This capability is crucial in determining the classification and character of lymphomas, such as diffuse large B-cell lymphoma or anaplastic T-cell lymphoma, at both the molecular and protein levels within the same tissue section. The EBER/IHC Dual Staining Kit thus offers a more comprehensive and accurate diagnostic approach, improving the identification and management of these complex hematological malignancies. The kit is available in two configurations: EBER Probe (ISH) + CD3 (IHC) Dual Staining Kit and EBER Probe (ISH) + CD20 (IHC) Dual Staining Kit, catering to the specific needs of different diagnostic scenarios.
By leveraging this dual-staining approach, pathologists can achieve highly reliable and reproducible results. The EBER/IHC Dual Staining Kit is crucial for diagnosing EBV-associated malignancies, including certain lymphomas and carcinomas. This enhanced diagnostic capability supports clinicians in making more informed decisions regarding patient management and therapy plans.
Future Trends in Molecular Diagnostics
Integrating Artificial Intelligence and Machine Learning
The integration of Artificial Intelligence (AI) and Machine Learning (ML) is poised to revolutionize molecular diagnostics. These technologies offer unparalleled capabilities in data analysis, pattern recognition, and predictive modeling. By incorporating AI and ML algorithms, diagnostic platforms can analyze vast datasets rapidly and with high precision, thereby improving diagnostic accuracy and efficiency.
AI-driven tools are particularly beneficial in areas such as image analysis for pathology, where they can assist in identifying abnormalities that might be overlooked by human eyes. Additionally, ML models can facilitate the interpretation of complex genomic data, aiding in the identification of novel biomarkers and elucidating disease mechanisms. The synergy between AI, ML, and molecular diagnostics promises to enhance diagnostic workflows and enable more personalized and predictive healthcare.
Expansion in Point-of-Care Testing
The growth of Point-of-Care Testing (POCT) represents another important trend in molecular diagnostics. POCT enables diagnostic tests to be performed at or near the location of patient care, delivering immediate results that are vital for prompt decision-making. The advent of portable and easy-to-use molecular diagnostic devices has made POCT more widely available across various clinical settings, such as outpatient clinics, emergency rooms, and even in remote areas.
The ability to perform rapid tests for infectious diseases, genetic conditions, and other biomarkers at the point of care reduces the time to diagnosis and treatment initiation. This immediate availability of diagnostic information not only enhances patient care but also helps in controlling disease outbreaks by enabling prompt intervention. The ongoing advancements in POCT technology are expected to further drive its adoption and utility in healthcare systems around the globe.
Development of Multi-Analyte Platforms
The development of multi-analyte platforms is an emerging frontier in molecular diagnostics. These platforms are designed to simultaneously detect and quantify multiple biomarkers from a single sample, offering a comprehensive overview of a patient’s molecular profile. Multi-analyte platforms leverage various technologies, including microarrays, multiplex PCR, and next-generation sequencing, to provide a holistic understanding of disease states.
This capability is particularly advantageous in oncology, where complex interactions between multiple genetic and protein markers need to be understood for accurate diagnosis and treatment planning. Multi-analyte platforms enable clinicians to gain insights into the molecular underpinnings of diseases, facilitating more precise and targeted therapeutic approaches. The integration of these platforms into routine clinical practice represents a significant step toward realizing the full potential of personalized medicine.
Summary
Molecular diagnostics is ushering in a new era in healthcare, marked by improved precision, early detection, and personalized treatment approaches. Leading companies such as Celnovte are at the cutting edge, offering groundbreaking tools that profoundly influence disease diagnosis and management. Emerging trends like AI integration, the growth of point-of-care testing (POCT), and the creation of multi-analyte platforms are anticipated to further boost the capabilities and reach of molecular diagnostics. These advancements promise better patient outcomes and a more efficient healthcare system. As these technologies progress, the future of molecular diagnostics appears extremely bright, set to transform our understanding and treatment of diseases.