Enhancing Bladder Cancer Care: The Role of Immunohistochemistry Biomarkers in Diagnosis and Management
2024-05-18
By celnovte
Bladder cancer remains a significant health concern worldwide, with a substantial impact on both men and women. Accurate diagnosis and effective management are critical for improving patient outcomes. In this blog, we explore the vital role of immunohistochemistry (IHC) biomarkers in bladder cancer, highlighting both established and emerging markers that are shaping the future of bladder cancer diagnostics and treatment.
Understanding Bladder Cancer
Bladder cancer is the seventh most common cancer among men in Brazil and accounts for a significant number of cancer-related deaths globally. The disease is primarily caused by environmental factors such as cigarette smoking, chemical exposure, and genetic predisposition. Pathological classification and staging of bladder cancer are crucial for determining prognosis and clinical management.
What is Immunohistochemistry (IHC)?
IHC is a widely used technique in cancer diagnostics. It involves staining tissue samples with specific antibodies to identify the presence of certain proteins that can indicate cancer. This method provides critical clinical information that aids in diagnosing and managing cancer.
Key IHC Biomarkers in Bladder Cancer
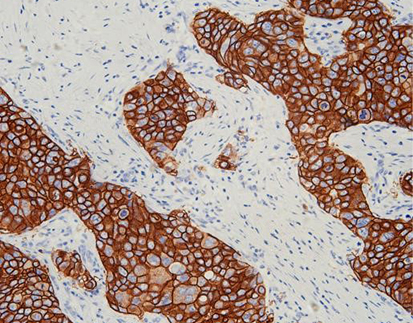
(1) Cytokeratins (CK5/6, CK14, CK20)
-
CK5/6 and CK14: These markers are expressed in basal cells and are associated with more aggressive bladder cancer subtypes.
-
CK20: Typically found in luminal cells, CK20 is associated with less aggressive forms of bladder cancer but is linked to higher recurrence rates.
(2) GATA3
-
GATA3 is a transcription factor and a luminal marker in bladder cancer. It is associated with better prognosis and higher recurrence-free survival rates.
(3) p53
-
The p53 tumor suppressor gene, when altered, can lead to tumor progression. Its expression patterns in bladder cancer are diverse, making it a complex but important marker.
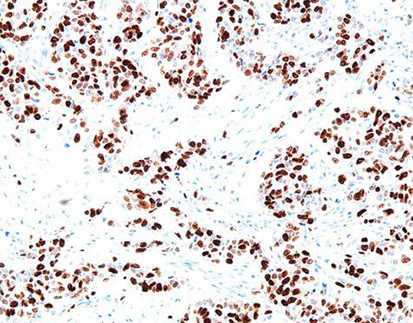
(4) Ki-67
-
Ki-67 is a proliferation marker that indicates the aggressiveness of cancer cells. Higher Ki-67 levels correlate with poorer outcomes and increased tumor aggressiveness.
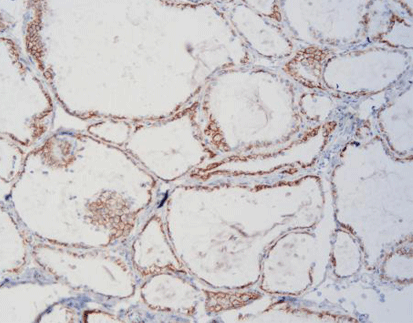
(5) Uroplakin II (UPII)
-
UPII is highly specific for urothelial carcinoma and helps distinguish bladder cancer from other malignancies.
Emerging IHC Biomarkers
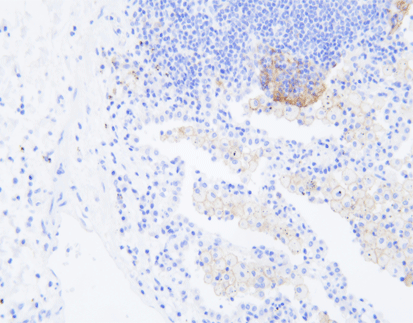
(1) HER-2
-
HER-2 is an oncogene that, when overexpressed, can lead to cancer progression. It shows promise as a target for personalized therapies in bladder cancer.
(2) PD-1 and PD-L1
-
These immune checkpoint proteins are critical targets for immunotherapy. Their expression in bladder cancer can indicate the potential effectiveness of immunotherapy treatments.
(3) E-cadherin
-
E-cadherin is involved in cell adhesion. Its downregulation is associated with increased cancer invasiveness and poorer prognosis.
(4) SOX2
-
SOX2 is a marker for cancer stem cells and is linked to poor prognosis and tumor progression.
(5) Vimentin
-
Vimentin is expressed in mesenchymal cells and is used to differentiate between muscle layers in bladder cancer, aiding in accurate staging and treatment planning.
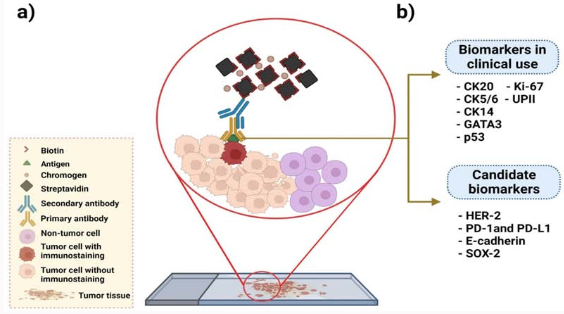
: a) Technique for immunohistochemistry using antibodies specific for tumor antigens and streptavidin [biotin complex. B) Proteins currently in clinical use
as predictive biomarkers in bladder cancer, as well as putative biomarkers examined in this work. Keratin 20 (CK20), cytokeratin 5/6 (CK5/6), keratin 14 (CK14),
GATA binding protein 3 (GATA3), tumor protein 53 (p53), phosphatase 1(interacting protein (Ki-67), and recombinant human uroplakin II (UPII) are clinically used
biomarkers, while candidate biomarkers include human epidermal growth factor receptor 2 (HER-2), programmed cell death protein 1 (PD-1), programmed deathligand 1 (PD-L1), epithelial cadherin (E-cadherin) and SRY-box transcription factor 2 (SOX-2).
The Importance of Accurate Biomarker Identification
Accurate identification and application of these biomarkers are essential for the effective management of bladder cancer. IHC is a cost-effective and widely accessible technique that can provide significant insights into tumor behavior and guide treatment decisions. However, ongoing research is necessary to refine the use of these biomarkers and explore new ones to enhance diagnostic precision and therapeutic outcomes.
Conclusion
The role of IHC biomarkers in bladder cancer diagnosis and management is indispensable. From established markers like cytokeratins and GATA3 to emerging ones like HER-2 and PD-L1, these biomarkers offer valuable information that can significantly influence clinical decisions and patient outcomes. As research progresses, the potential for new biomarkers continues to grow, promising even more precise and effective cancer diagnostics and treatments in the future.
Stay informed about the latest developments in bladder cancer biomarkers and their clinical applications by following our updates.
For more detailed insights and research findings, visit Celnovte Biotech’s Website.
About Celnovte Biotech:
Celnovte Biotech specializes in the research, development, manufacturing, and distribution of advanced pathological diagnostic reagents and instruments. Our market-leading portfolio includes IHC, CISH, and FISH products, designed to meet the highest standards of sensitivity and specificity.
-
Primary Antibody: 100+ Self-cloned MMab and RMab primary antibodies won optimal NordiQC assessment.
-
MicroStacker™ IHC Detection System: Renowned for its unsurpassed sensitivity and specificity.
-
PolyStacker™ Technology: Reduces turnaround time of frozen section IHC experiments to as short as 10 minutes.
-
SuperISH™ RNA In-Situ Hybridization Technology: Enables detection of RNA targets at the single molecular level and single-cell resolution.
-
Automated Instrumentation: Including IHC slide stainers, H&E stainers, special stainers, cytopathology instruments, and digital slide scanners. Since 2018, we have successfully installed over 600 units of fully automated IHC stainers globally.
Opportunity for Regional Distributors:
We are actively looking for regional distributors in Indonesia. If you are interested or know someone who might be, please get in touch with us during the conference or reply this email.
Stay Connected:
-
Explore the latest in pathology innovations on our LinkedIn Page.
-
Check out our brochures here.
-
Follow our facebook for the news: Facebook Page.