PRODUCT CATEGORY
PRODUCT CATEGORY
CONTACT US
ETV6/NTRK3 Fusion Gene t (12;15) Probe Reagent (Fluorescence in Situ Hybridization)
Clinic Signification:
1. Vitrakvi is the first FDA-approved oral TRK inhibitor to be marketed and also the first "broad-spectrum" anticancer drug that has nothing to do with tumor type. Targeted therapy for patients with NTRK gene (NTRK1/2/3) fusion , including: lung cancer, thyroid cancer, melanoma, gastrointestinal cancer, colon cancer, soft tissue sarcoma, salivary glands, infantile fibrosarcoma, appendix cancer, breast cancer, cholangiocarcinoma, pancreatic cancer etc.
Contrast
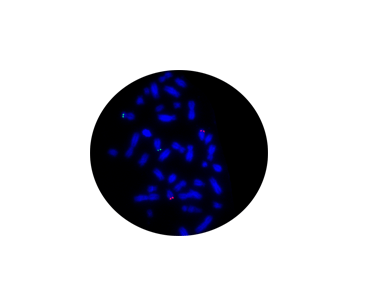
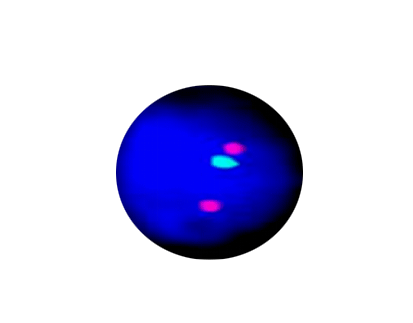
NTRK3/ETV6 Fusion
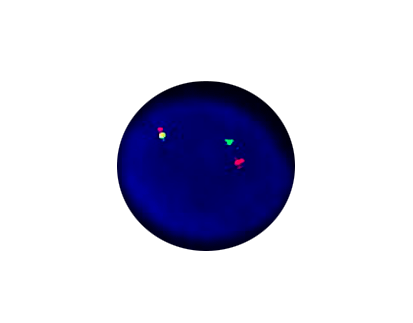
ETV6 Breakpart
More Info
Probe Description: ETV6/NTRK3
Cat.No.: CF1078
Specification: 5 tests/box;10 tests/box, 20 tests/box
Related Product







 PRODUCT CATEGORY
PRODUCT CATEGORY






 Chat
Chat

 message
message

 Quote
Quote